The clinical implications and molecular features of intrahepatic cholangiocarcinoma with perineural invasion
- PMID: 36418844
- PMCID: PMC9895046
- DOI: 10.1007/s12072-022-10445-1
The clinical implications and molecular features of intrahepatic cholangiocarcinoma with perineural invasion
Abstract
Background: Perineural invasion (PNI) is associated with metastasis in malignancies, including intrahepatic cholangiocarcinoma (ICC), and is correlated with poor prognosis.
Methods: The study included three large cohorts: ZS-ICC and TMA cohorts from our team, MSK cohort from a public database, and a small cohort named cohort 4. Prognostic implications of PNI were investigated in MSK cohort and TMA cohort. PNI-related genomic and transcriptomic profiles were analyzed in MSK and ZS-ICC cohorts. GO, KEGG, and ssGSEA analyses were performed. Immunohistochemistry was used to investigate the relationship between PNI and markers of neurons, hydrolases, and immune cells. The efficacy of adjuvant therapy in ICC patients with PNI was also assessed.
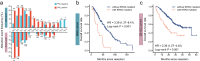
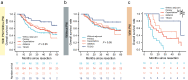
Results: A total of 30.6% and 20.7% ICC patients had PNI in MSK and TMA cohorts respectively. Patients with PNI presented with malignant phenotypes such as high CA19-9, the large bile duct type, lymph node invasion, and shortened overall survival (OS) and relapse-free survival (RFS). Nerves involved in PNI positively express tyrosine hydroxylase (TH), a marker of sympathetic nerves. Patients with PNI showed high mutation frequency of KRAS and an immune suppressive metastasis prone niche of decreased NK cell, increased neutrophil, and elevated PD-L1, CD80, and CD86 expression. Patients with PNI had an extended OS after adjuvant therapy with TEGIO, GEMOX, or capecitabine.
Conclusion: Our study deciphered the genomic features and the immune suppressive metastasis-prone niche in ICC with PNI. Patients with PNI showed a poor prognosis after surgery but a good response to adjuvant chemotherapy.
Keywords: Adjuvant therapy; Bioinformatics; Intrahepatic cholangiocarcinoma; KRAS; Metastasis prone niche; Overall survival; Pathology feature; Perineural invasion; Relapse-free survival; Sympathetic nerve.
© 2022. The Author(s).
Conflict of interest statement
Xian-Long Meng, Jia-Cheng Lu, Hai-Ying Zeng, Zhen Chen, Xiao-Jun Guo, Chao Gao, Yan-Zi Pei, Shu-Yang Hu, Mu Ye, Qi-Man Sun, Guo-Huang Yang, Jia-Bin Cai, Pei-Xin Huang, Lei Yv, Lv Zhang, Ying-Hong Shi, Ai-Wu Ke, Jian Zhou, Jia Fan, Yi Chen, Xiao-Yong Huang, Guo-Ming Shi have declared that no competing interest exists.
Figures


References
-
- Demir IE, Friess H, Ceyhan GO. Neural plasticity in pancreatitis and pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2015;12(11):649–659. - PubMed
MeSH terms
Grants and funding
- SHDC‑2020CR1003A/the Clinical Research Plan of SHDC
- XHLHGG202103/the Key Disease Joint Research Program of Xuhui District
- 2019YFC1316000/Key Technologies Research and Development Program
- 81972232/Innovative Research Group Project of the National Natural Science Foundation of China
- 20JC1419103/the Shanghai Municipal Natural Science Foundation
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

