Acute gut inflammation reduces neural activity and spine maturity in hippocampus but not basolateral amygdala
- PMID: 36418891
- PMCID: PMC9684565
- DOI: 10.1038/s41598-022-24245-y
Acute gut inflammation reduces neural activity and spine maturity in hippocampus but not basolateral amygdala
Abstract
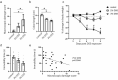
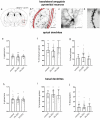
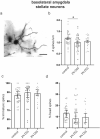
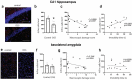
Gastrointestinal tract (gut) inflammation increases stress and threat-coping behaviors, which are associated with altered activity in fear-related neural circuits, such as the basolateral amygdala and hippocampus. It remains to be determined whether inflammation from the gut affects neural activity by altering dendritic spines. We hypothesized that acute inflammation alters dendritic spines in a brain region-specific manner. Here we show that acute gut inflammation (colitis) evoked by dextran sodium sulfate (DSS) did not affect the overall spine density in the CA1 region of hippocampus, but increased the relative proportion of immature spines to mature spines on basal dendrites of pyramidal neurons. In contrast, in animals with colitis, no changes in spine density or composition on dendrites of pyramidal cells was observed in the basolateral amygdala. Rather, we observed decreased spine density on dendrites of stellate neurons, but not the relative proportions of mature vs immature spines. We used cFos expression evoked by the forced swim task as a measure of neural activity during stress and found no effect of DSS on the density of cFos immunoreactive neurons in basolateral amygdala. In contrast, fewer CA1 neurons expressed cFos in mice with colitis, relative to controls. Furthermore, CA1 cFos expression negatively correlated with active stress-coping in the swim task and was negatively correlated with gut inflammation. These data reveal that the effects of acute gut inflammation on synaptic remodeling depend on brain region, neuronal phenotype, and dendrite location. In the hippocampus, a shift to immature spines and hypoactivity are more strongly related to colitis-evoked behavioral changes than is remodeling in basolateral amygdala.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Neuendorf R, Harding A, Stello N, Hanes D, Wahbeh H. Depression and anxiety in patients with inflammatory bowel disease: A systematic review. J. Psychosom. Res. 2016;87:70–80. - PubMed
-
- Barberio B, Zamani M, Black CJ, Savarino EV, Ford AC. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021;6:359–370. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

