Modular automated microfluidic cell culture platform reduces glycolytic stress in cerebral cortex organoids
- PMID: 36418910
- PMCID: PMC9684529
- DOI: 10.1038/s41598-022-20096-9
Modular automated microfluidic cell culture platform reduces glycolytic stress in cerebral cortex organoids
Abstract
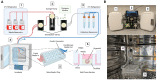
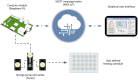
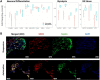
Organ-on-a-chip systems combine microfluidics, cell biology, and tissue engineering to culture 3D organ-specific in vitro models that recapitulate the biology and physiology of their in vivo counterparts. Here, we have developed a multiplex platform that automates the culture of individual organoids in isolated microenvironments at user-defined media flow rates. Programmable workflows allow the use of multiple reagent reservoirs that may be applied to direct differentiation, study temporal variables, and grow cultures long term. Novel techniques in polydimethylsiloxane (PDMS) chip fabrication are described here that enable features on the upper and lower planes of a single PDMS substrate. RNA sequencing (RNA-seq) analysis of automated cerebral cortex organoid cultures shows benefits in reducing glycolytic and endoplasmic reticulum stress compared to conventional in vitro cell cultures.
© 2022. The Author(s).
Conflict of interest statement
S.T.S and G.L.M. are founders of OrganOmics, a company that may be affected by the research reported in the enclosed paper. All other authors declare no competing interests.
Figures




References
-
- Gey GO, Coffman WD, Kubicek MT. Tissue culture studies of the proliferative capacity of cervical carcinoma and normal epithelium. Cancer Res. 1952;12:264–265.
-
- Scherer WF, Syverton JT, Gey GO. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain hela) derived from an epidermoid carcinoma of the cervix. Exp. Med. 1953;97:695–710. doi: 10.1084/jem.97.5.695. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

