Convalescent or standard plasma versus standard of care in the treatment of COVID-19 patients with respiratory impairment: short and long-term effects. A three-arm randomized controlled clinical trial
- PMID: 36418984
- PMCID: PMC9682750
- DOI: 10.1186/s12879-022-07716-5
Convalescent or standard plasma versus standard of care in the treatment of COVID-19 patients with respiratory impairment: short and long-term effects. A three-arm randomized controlled clinical trial
Abstract
Background: The efficacy of early treatment with convalescent plasma in patients with COVID-19 is debated. Nothing is known about the potential effect of other plasma components other than anti-SARS-CoV-2 antibodies.
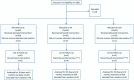
Methods: To determine whether convalescent or standard plasma would improve outcomes for adults in early phase of Covid19 respiratory impairment we designed this randomized, three-arms, clinical trial (PLACO COVID) blinded on interventional arms that was conducted from June 2020 to August 2021. It was a multicentric trial at 19 Italian hospitals. We enrolled 180 hospitalized adult patients with COVID-19 pneumonia within 5 days from the onset of respiratory distress. Patients were randomly assigned in a 1:1:1 ratio to standard of care (n = 60) or standard of care + three units of standard plasma (n = 60) or standard of care + three units of high-titre convalescent plasma (n = 60) administered on days 1, 3, 5 after randomization. Primary outcome was 30-days mortality. Secondary outcomes were: incidence of mechanical ventilation or death at day 30, 6-month mortality, proportion of days with mechanical ventilation on total length of hospital stay, IgG anti-SARS-CoV-2 seroconversion, viral clearance from plasma and respiratory tract samples, and variations in Sequential Organ Failure Assessment score. The trial was analysed according to the intention-to-treat principle.
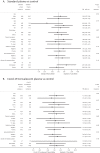
Results: 180 patients (133/180 [73.9%] males, mean age 66.6 years [IQR 57-73]) were enrolled a median of 8 days from onset of symptoms. At enrollment, 88.9% of patients showed moderate/severe respiratory failure. 30-days mortality was 20% in Control arm, 23% in Convalescent (risk ratio [RR] 1.13; 95% confidence interval [CI], 0.61-2.13, P = 0.694) and 25% in Standard plasma (RR 1.23; 95%CI, 0.63-2.37, P = 0.544). Time to viral clearance from respiratory tract was 21 days for Convalescent, 28 for Standard plasma and 23 in Control arm but differences were not statistically significant. No differences for other secondary endpoints were seen in the three arms. Serious adverse events were reported in 1.7%, 3.3% and 5% of patients in Control, Standard and Convalescent plasma arms respectively.
Conclusions: Neither high-titer Convalescent nor Standard plasma improve outcomes of COVID-19 patients with acute respiratory failure. Trial Registration Clinicaltrials.gov Identifier: NCT04428021. First posted: 11/06/2020.
Keywords: COVID-19 convalescent plasma; COVID-19 outcomes; COVID-19 therapy; Randomized clinical trial.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

