Neonatal outcomes from a quasi-experimental clinical trial of Family Integrated Care versus Family-Centered Care for preterm infants in U.S. NICUs
- PMID: 36418988
- PMCID: PMC9682629
- DOI: 10.1186/s12887-022-03732-1
Neonatal outcomes from a quasi-experimental clinical trial of Family Integrated Care versus Family-Centered Care for preterm infants in U.S. NICUs
Abstract
Background: Family Integrated Care (FICare) benefits preterm infants compared with Family-Centered Care (FCC), but research is lacking in United States (US) Neonatal Intensive Care Units (NICUs). The outcomes for infants of implementing FICare in the US are unknown given differences in parental leave benefits and health care delivery between the US and other countries where FICare is used. We compared preterm weight and discharge outcomes between FCC and mobile-enhanced FICare (mFICare) in the US.
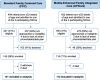
Methods: In this quasi-experimental study, we enrolled preterm infant (≤ 33 weeks)/parent dyads from 3 NICUs into sequential cohorts: FCC or mFICare. Our primary outcome was 21-day change in weight z-scores. Our secondary outcomes were nosocomial infection, bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), and human milk feeding (HMF) at discharge. We used intention-to-treat analyses to examine the effect of the FCC and mFICare models overall and per protocol analyses to examine the effects of the mFICare intervention components.
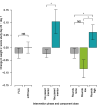
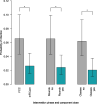
Findings: 253 infant/parent dyads participated (141 FCC; 112 mFICare). There were no parent-related adverse events in either group. In intention-to-treat analyses, we found no group differences in weight, ROP, BPD or HMF. The FCC cohort had 2.6-times (95% CI: 1.0, 6.7) higher odds of nosocomial infection than the mFICare cohort. In per-protocol analyses, we found that infants whose parents did not receive parent mentoring or participate in rounds lost more weight relative to age-based norms (group-difference=-0.128, CI: -0.227, -0.030; group-difference=-0.084, CI: -0.154, -0.015, respectively). Infants whose parents did not participate in rounds or group education had 2.9-times (CI: 1.0, 9.1) and 3.8-times (CI: 1.2, 14.3) higher odds of nosocomial infection, respectively.
Conclusion: We found indications that mFICare may have direct benefits on infant outcomes such as weight gain and nosocomial infection. Future studies using implementation science designs are needed to optimize intervention delivery and determine acute and long-term infant and family outcomes.
Clinical trial registration: NCT03418870 01/02/2018.
Keywords: Clinical rounds; Family partnerships; Infant; Neonatology; Nosocomial infection; Parent education; Peer mentors; Weight gain.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Maternal mental health after infant discharge: a quasi-experimental clinical trial of family integrated care versus family-centered care for preterm infants in U.S. NICUs.BMC Pediatr. 2023 Aug 10;23(1):396. doi: 10.1186/s12887-023-04211-x. BMC Pediatr. 2023. PMID: 37563722 Free PMC article. Clinical Trial.
-
Comparison of family centered care with family integrated care and mobile technology (mFICare) on preterm infant and family outcomes: a multi-site quasi-experimental clinical trial protocol.BMC Pediatr. 2019 Dec 2;19(1):469. doi: 10.1186/s12887-019-1838-3. BMC Pediatr. 2019. PMID: 31791285 Free PMC article.
-
Mobile-enhanced Family Integrated Care for preterm infants: A qualitative study of parents' views.PEC Innov. 2024 Apr 30;4:100284. doi: 10.1016/j.pecinn.2024.100284. eCollection 2024 Dec. PEC Innov. 2024. PMID: 38737891 Free PMC article.
-
Family Integrated Care for Preterm Infants.Crit Care Nurs Clin North Am. 2020 Jun;32(2):149-165. doi: 10.1016/j.cnc.2020.01.001. Epub 2020 Mar 31. Crit Care Nurs Clin North Am. 2020. PMID: 32402313 Review.
-
Effects of family-centred care interventions on preterm infants and parents in neonatal intensive care units: A systematic review and meta-analysis of randomised controlled trials.Aust Crit Care. 2019 Jan;32(1):63-75. doi: 10.1016/j.aucc.2018.10.007. Epub 2018 Dec 13. Aust Crit Care. 2019. PMID: 30554939
Cited by
-
Evaluation of a Model of Transitional Care After Preterm Birth on Parents' Mental Health and Self-Efficacy: A Randomized Controlled Pilot Trial.Children (Basel). 2024 Oct 18;11(10):1260. doi: 10.3390/children11101260. Children (Basel). 2024. PMID: 39457225 Free PMC article.
-
Parental participation in newborn care in the view of health care providers in Uganda: a qualitative study.Reprod Health. 2024 Oct 29;21(1):155. doi: 10.1186/s12978-024-01896-w. Reprod Health. 2024. PMID: 39472919 Free PMC article.
-
Effect of family integrated care on breastfeeding of preterm infants: A scoping review.Nurs Open. 2023 Sep;10(9):5950-5960. doi: 10.1002/nop2.1888. Epub 2023 Jun 12. Nurs Open. 2023. PMID: 37306324 Free PMC article.
-
Maternal mental health after infant discharge: a quasi-experimental clinical trial of family integrated care versus family-centered care for preterm infants in U.S. NICUs.BMC Pediatr. 2023 Aug 10;23(1):396. doi: 10.1186/s12887-023-04211-x. BMC Pediatr. 2023. PMID: 37563722 Free PMC article. Clinical Trial.
-
Parents' Experiences With an Early Behavioral Intervention, H-HOPE, in the NICU and at Home: A Qualitative Study.Adv Neonatal Care. 2025 Aug 1;25(4):401-410. doi: 10.1097/ANC.0000000000001282. Epub 2025 Jul 30. Adv Neonatal Care. 2025. PMID: 40609008 Free PMC article.
References
-
- World Health Organization. Survive and thrive: transforming care for every small and sick newborn. [Internet]. World Health Organization; Geneva; 2019 [Cited 2022 Apr 11]. Available from: https://www.who.int/publications/i/item/9789241515887.

