Hepatitis C prevalence in incarcerated settings between 2013-2021: a systematic review and meta-analysis
- PMID: 36419013
- PMCID: PMC9685883
- DOI: 10.1186/s12889-022-14623-6
Hepatitis C prevalence in incarcerated settings between 2013-2021: a systematic review and meta-analysis
Abstract
Background: The introduction of highly effective direct-acting antiviral therapy has changed the hepatitis C virus (HCV) treatment paradigm. However, a recent update on HCV epidemiology in incarcerated settings is necessary to accurately determine the extent of the problem, provide information to policymakers and public healthcare, and meet the World Health Organization's goals by 2030. This systematic review and meta-analysis were performed to determine the prevalence of HCV Ab and RNA in incarcerated settings.
Methods: For this systematic review and meta-analysis, we searched PubMed, Embase, Scopus, and Web of Science for papers published between January 2013 and August 2021. We included studies with information on the prevalence of HCV Ab or RNA in incarcerated settings. A random-effects meta-analysis was done to calculate the pooled prevalence and meta-regression to explore heterogeneity.
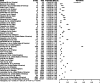
Results: Ninety-two unique sources reporting data for 36 countries were included. The estimated prevalence of HCV Ab ranged from 0.3% to 74.4%. HCV RNA prevalence (available in 46 sources) ranged from 0% to 56.3%. Genotypes (available in 19 sources) 1(a) and 3 were most frequently reported in incarcerated settings. HCV/HIV coinfection (available in 36 sources) was highest in Italy, Estonia, Pakistan, and Spain. Statistical analysis revealed that almost all observed heterogeneity reflects real differences in prevalence between studies, considering I2 was very high in the meta-analysis.
Conclusions: HCV in incarcerated settings is still a significant problem with a higher prevalence than in the general population. It is of utmost importance to start screening for HCV (Ab and RNA) in incarcerated settings to give clear, reliable and recent figures to plan further treatment. This is all in the context of meeting the 2030 WHO targets which are only less than a decade away.
Trial registration: PROSPERO: CRD42020162616.
Keywords: Global health; Hepatitis c; Incarcerated setting; Meta-analysis; Prevalence.
© 2022. The Author(s).
Conflict of interest statement
D.B. has received travel grants from AbbVie and Gilead Sciences; R.B. has received travel grants from AbbVie, Gilead Sciences and Merck Sharp & Dohme (MSD) and research grants from Gilead and MSD; O.K. has received a travel grant from Gilead Sciences and his institution received research grants from Gilead Sciences and CyTuVax BV; F.N. has received research grants, consultancy agreements and travel grants from UCB, Ipsen, Roche, Astellas, Ferring, Novartis, Janssen-Cilag, Abbvie, Gilead, CAF, Intercept, Gore, Bristol-Myers Squibb (BMS), MSD, Promethera Biosciences, Ono Pharma, Durect; N.H. reports grants from GlaxoSmithKline, grants from Johnson & Johnson pharmaceuticals and grants from Pfizer; G.R. has received research grants from AbbVie, Janssen Pharmaceuticals, MSD, and consultancy agreements for AbbVie, BMS, Gilead Sciences and MSD. All other co-authors report no competing interests.
Figures



References
-
- World Health Organization. Global Hepatitis Report, 2017. WHO: Geneva, April, 2017. http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf.
-
- Wright N, Reimer J, Somaini L, Roncero C, Maremmani I, Simon N, Krajci P, Littlewood R, D'Agnone O, Alho H, et al. Are we ready to treat hepatitis C virus in individuals with opioid use disorder: assessment of readiness in European countries on the basis of an expert-generated model. Eur J Gastroenterol Hepatol. 2017;29(11):1206–1214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

