Synthesis, biological evaluation, and computational studies of some novel quinazoline derivatives as anticancer agents
- PMID: 36419100
- PMCID: PMC9682696
- DOI: 10.1186/s13065-022-00893-z
Synthesis, biological evaluation, and computational studies of some novel quinazoline derivatives as anticancer agents
Abstract
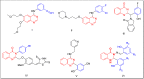
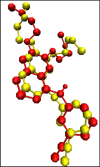
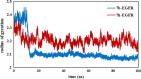
A series of quinazolinone derivatives (7a-7h) were synthesized as antiproliferative agents. All compounds, were synthesized through three steps method and structurally evaluated by FTIR, 1H-NMR, 13CNMR and Mass spectroscopy. Their cytotoxic activities were assessed using MTT protocol against three humans cancerous (MCF-7, A549 and 5637) and normal (MRC-5) cell lines. In addition, molecular docking and simulation studies of the synthesized compounds were performed to assessment their orientation, interaction mode against EGFR as plausible mechanism of quinazoline compounds as anticancer agents. The synthesized compounds mostly showed moderate activity against the three studied cell lines. They also indicated an appropriate selectivity against tumorigenic and non-tumorigenic cell line. The molecular docking results also confirmed biological activity. Most of the compounds fulfilled Lipinski rule. Collectively, these compounds with further modification can be considered as potent antiproliferative agents.
Keywords: Anticancer agents; Computational Studies; Quinazoline; Synthesis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
6-Bromo quinazoline derivatives as cytotoxic agents: design, synthesis, molecular docking and MD simulation.BMC Chem. 2024 Jul 4;18(1):125. doi: 10.1186/s13065-024-01230-2. BMC Chem. 2024. PMID: 38965630 Free PMC article.
-
Design, synthesis, computational study and cytotoxic evaluation of some new quinazoline derivatives containing pyrimidine moiety.Sci Rep. 2023 Sep 2;13(1):14461. doi: 10.1038/s41598-023-41530-6. Sci Rep. 2023. PMID: 37660139 Free PMC article.
-
Design, synthesis and evaluation of novel 1,2,4-triazole derivatives as promising anticancer agents.BMC Chem. 2022 Nov 12;16(1):91. doi: 10.1186/s13065-022-00887-x. BMC Chem. 2022. PMID: 36369166 Free PMC article.
-
Synthesis of some novel dibromo-2-arylquinazolinone derivatives as cytotoxic agents.Res Pharm Sci. 2019 Mar 8;14(2):115-121. doi: 10.4103/1735-5362.253358. eCollection 2019 Apr. Res Pharm Sci. 2019. PMID: 31620187 Free PMC article.
-
An insight into the therapeutic potential of quinazoline derivatives as anticancer agents.Medchemcomm. 2017 Apr 7;8(5):871-885. doi: 10.1039/c7md00097a. eCollection 2017 May 1. Medchemcomm. 2017. PMID: 30108803 Free PMC article. Review.
Cited by
-
Design, synthesis, in-silico studies and apoptotic activity of novel amide enriched 2-(1H)- quinazolinone derivatives.Heliyon. 2024 Apr 26;10(9):e30292. doi: 10.1016/j.heliyon.2024.e30292. eCollection 2024 May 15. Heliyon. 2024. PMID: 38711664 Free PMC article.
-
6-Bromo quinazoline derivatives as cytotoxic agents: design, synthesis, molecular docking and MD simulation.BMC Chem. 2024 Jul 4;18(1):125. doi: 10.1186/s13065-024-01230-2. BMC Chem. 2024. PMID: 38965630 Free PMC article.
-
Design, synthesis, biological assessments and computational studies of 3-substituted phenyl quinazolinone derivatives as promising anti-cancer agents.BMC Chem. 2025 May 13;19(1):125. doi: 10.1186/s13065-025-01492-4. BMC Chem. 2025. PMID: 40361154 Free PMC article.
-
Synthesis, design, biological evaluation, and computational analysis of some novel uracil-azole derivatives as cytotoxic agents.BMC Chem. 2024 Jan 3;18(1):3. doi: 10.1186/s13065-023-01106-x. BMC Chem. 2024. PMID: 38173035 Free PMC article.
-
Design and Synthesis of 1-(4-Bromo-2-(Pyrrolidine-1-Yl) Benzyl) Piperidine-based Derivatives as Anti-tubulin Agents.Curr Top Med Chem. 2025;25(11):1389-1402. doi: 10.2174/0115680266336578241114072129. Curr Top Med Chem. 2025. PMID: 39757672
References
-
- Shao Z, Jahanbani A, Sheikholeslami SM. Multiplicative topological indices of molecular structure in anticancer drugs. Polycyclic Aromat Compd. 2022;42(2):475–488. doi: 10.1080/10406638.2020.1743329. - DOI
-
- Mahmoud HK, Gomha SM, Farghaly TA, Awad HM. Synthesis of thiazole linked imidazo [2, 1-b] thiazoles as anticancer agents. Polycyclic Aromat Compd. 2021;41(8):1608–1622. doi: 10.1080/10406638.2019.1689514. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
