The use of two Comfort Young Child Formulas in the dietary management of toddlers with functional constipation: a randomized controlled trial
- PMID: 36419103
- PMCID: PMC9682631
- DOI: 10.1186/s12887-022-03725-0
The use of two Comfort Young Child Formulas in the dietary management of toddlers with functional constipation: a randomized controlled trial
Abstract
Background: Pharmacological intervention with laxatives is the conventional treatment for functional constipation (FC). Data to support the dietary management of FC is lacking. This study compared the efficacy of two Comfort young child formulas (YCFs) with regards to the maintenance of healthy stooling parameters in toddlers with a history of constipation. It was registered in the Netherlands Trial Registry [identifier: NL7420 (NTR7653)], registration date 20/09/2018.
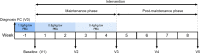
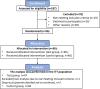
Methods: Ninety-five healthy toddlers, aged 12 to 32 months, diagnosed with FC (Rome III criteria) were randomized to receive one of two study formulas after pharmacological treatment. For the first month of the intervention, subjects received a laxative in a decreasing maintenance dose alongside a test or control formula (maintenance phase). Subsequently, subjects only consumed formula for another month (post-maintenance phase). Stooling parameters were obtained weekly using the Bristol Stool Scale and the modified Rome III Questionnaire on Paediatric Gastrointestinal Symptoms for infants and toddlers. Differences in percentages of hard stools (primary outcome) and other stooling parameters were analysed using analysis of covariance and Chi-Square methods.
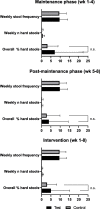
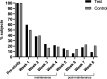
Results: Both formulas resulted in similar overall percentage of hard stools during the intervention period, respectively 5.02% in the test and 2.99% in the control group (n.s.). In the test group, percentages dropped from 7.11% at the end of the maintenance phase, to 3.92% at the end of the post-maintenance phase. In contrast, the percentage of hard stools in the control group was similar at the end of the maintenance (3.18%) and post-maintenance phase (2.83%; n.s.). No difference was found in the overall stool frequency between groups. At the end of the maintenance phase, only 22% and 19% of toddlers consuming the test and control formulae, respectively, met 2 or more of the criteria for FC. At the end of the study, this percentage of subjects decreased further to 9% in the test group, which tended to be lower compared to the 21% found in the control (p = 0.107). No laxative use was reported in either study group during the post-maintenance phase.
Conclusion: Both Comfort YCF support the maintenance of improved stooling over time in toddlers with a history of constipation. The percentage of subjects suffering from functional constipation tended to be lower after the intervention period when receiving the formula with intact protein.
Keywords: Dietary management; Functional constipation; Hard stools; Intact protein; Young child formula.
© 2022. The Author(s).
Conflict of interest statement
Denise Hofman, Miriam Contreras, Urszula Kudla, Eva Kontopodi, and Jeske Hageman are employees of FrieslandCampina at the time of manuscript creation. Daniel Alfonso Cisneros Sevilla, Darelia Alelí Topete Ángel, Sergio Díaz Madero, and Joshué David Covarrubias Esquer report no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

