Innate immunity, cytokine storm, and inflammatory cell death in COVID-19
- PMID: 36419185
- PMCID: PMC9682745
- DOI: 10.1186/s12967-022-03767-z
Innate immunity, cytokine storm, and inflammatory cell death in COVID-19
Abstract
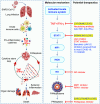
The innate immune system serves as the first line of defense against invading pathogens; however, dysregulated innate immune responses can induce aberrant inflammation that is detrimental to the host. Therefore, careful innate immune regulation is critical during infections. The coronavirus disease 2019 (COVID-19) pandemic is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and has resulted in global morbidity and mortality as well as socio-economic stresses. Innate immune sensing of SARS-CoV-2 by multiple host cell pattern recognition receptors leads to the production of various pro-inflammatory cytokines and the induction of inflammatory cell death. These processes can contribute to cytokine storm, tissue damage, and acute respiratory distress syndrome. Here, we discuss the sensing of SARS-CoV-2 to induce innate immune activation and the contribution of this innate immune signaling in the development and severity of COVID-19. In addition, we provide a conceptual framework for innate immunity driving cytokine storm and organ damage in patients with severe COVID-19. A better understanding of the molecular mechanisms regulated by innate immunity is needed for the development of targeted modalities that can improve patient outcomes by mitigating severe disease.
Keywords: IFN; Necroptosis; PANoptosis; PANoptosome; Pyroptosis; TNF.
© 2022. The Author(s).
Conflict of interest statement
T.-D.K. is a consultant for Pfizer.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

