Neuroprotective effects of a lead compound from coral via modulation of the orphan nuclear receptor Nurr1
- PMID: 36419251
- PMCID: PMC9928544
- DOI: 10.1111/cns.14025
Neuroprotective effects of a lead compound from coral via modulation of the orphan nuclear receptor Nurr1
Abstract
Aims: To screen coral-derived compounds with neuroprotective activity and clarify the potential mechanism of lead compounds.
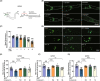
Methods: The lead compounds with neuroprotective effects were screened by H2 O2 and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPP+ )-induced cell damage models in SH-SY5Y cells. CCK8 and LDH assays were used to detect cell viability. The anti-apoptosis of lead compounds was evaluated by flow cytometry. JC-1 and MitoSox assays were performed to examine the changes in mitochondrial membrane potential and mitochondrial ROS level. Survival of primary cortical and dopaminergic midbrain neurons was measured by MAP2 and TH immunoreactivities. The Caenorhabditis elegans (C. elegans) model was established to determine the effect of lead compounds on dopaminergic neurons and behavior changes.
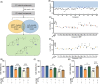
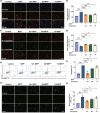
Results: Three compounds (No. 63, 68, and 74), derived from marine corals, could markedly alleviate the cell damage and notably reverse the loss of worm dopaminergic neurons. Further investigation indicated that compound 63 could promote the expression of Nurr1 and inhibit neuronal apoptosis signaling pathways.
Conclusion: Lead compounds from marine corals exerted significant neuroprotective effects, which indicated that coral might be a new and potential resource for screening and isolating novel natural compounds with neuroprotective effects. Furthermore, this study also provided a new strategy for the clinical treatment of neurodegenerative diseases such as Parkinson's disease.
Keywords: C. elegans; Nurr1; SH-SY5Y cells; corals-derived compounds; neuroprotection.
© 2022 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
All the authors declare no conflict of interest.
Figures




Similar articles
-
Nurr1 overexpression exerts neuroprotective and anti-inflammatory roles via down-regulating CCL2 expression in both in vivo and in vitro Parkinson's disease models.Biochem Biophys Res Commun. 2017 Jan 22;482(4):1312-1319. doi: 10.1016/j.bbrc.2016.12.034. Epub 2016 Dec 8. Biochem Biophys Res Commun. 2017. PMID: 27940361
-
Artemisia Leaf Extract protects against neuron toxicity by TRPML1 activation and promoting autophagy/mitophagy clearance in both in vitro and in vivo models of MPP+/MPTP-induced Parkinson's disease.Phytomedicine. 2022 Sep;104:154250. doi: 10.1016/j.phymed.2022.154250. Epub 2022 Jun 15. Phytomedicine. 2022. PMID: 35752074
-
A novel synthetic activator of Nurr1 induces dopaminergic gene expression and protects against 6-hydroxydopamine neurotoxicity in vitro.Neurosci Lett. 2015 Oct 21;607:83-89. doi: 10.1016/j.neulet.2015.09.015. Epub 2015 Sep 14. Neurosci Lett. 2015. PMID: 26383113 Free PMC article.
-
Equol, a Blood-Brain Barrier Permeable Gut Microbial Metabolite of Dietary Isoflavone Daidzein, Exhibits Neuroprotective Effects against Neurotoxins Induced Toxicity in Human Neuroblastoma SH-SY5Y Cells and Caenorhabditis elegans.Plant Foods Hum Nutr. 2020 Dec;75(4):512-517. doi: 10.1007/s11130-020-00840-0. Plant Foods Hum Nutr. 2020. PMID: 32761299
-
SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson's disease.Chin Med J (Engl). 2010 Apr 20;123(8):1086-92. Chin Med J (Engl). 2010. PMID: 20497720 Review.
Cited by
-
Oxidative Stress, Oxidative Damage, and Cell Apoptosis: Toxicity Induced by Arecoline in Caenorhabditis elegans and Screening of Mitigating Agents.Toxins (Basel). 2024 Aug 12;16(8):352. doi: 10.3390/toxins16080352. Toxins (Basel). 2024. PMID: 39195762 Free PMC article.
References
-
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10:S10‐S17. - PubMed
-
- Vazquez‐Velez GE, Zoghbi HY. Parkinson's disease genetics and pathophysiology. Annu Rev Neurosci. 2021;44:87‐108. - PubMed
-
- Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2020;27(1):27‐42. - PubMed
-
- Srivastava AK, Roy Choudhury S, Karmakar S. Melatonin/polydopamine nanostructures for collective neuroprotection‐based Parkinson's disease therapy. Biomater Sci. 2020;8(5):1345‐1363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

