Rectal Culture-Based Versus Empirical Antibiotic Prophylaxis to Prevent Infectious Complications in Men Undergoing Transrectal Prostate Biopsy: A Randomized, Nonblinded Multicenter Trial
- PMID: 36419331
- PMCID: PMC10069853
- DOI: 10.1093/cid/ciac913
Rectal Culture-Based Versus Empirical Antibiotic Prophylaxis to Prevent Infectious Complications in Men Undergoing Transrectal Prostate Biopsy: A Randomized, Nonblinded Multicenter Trial
Abstract
Background: An increase in infections after transrectal prostate biopsy (PB), related to an increasing number of patients with ciprofloxacin-resistant rectal flora, necessitates the exploration of alternatives for the traditionally used empirical prophylaxis of ciprofloxacin. We compared infectious complication rates after transrectal PB using empirical ciprofloxacin prophylaxis versus culture-based prophylaxis.
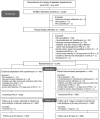
Methods: In this nonblinded, randomized trial, between 4 April 2018 and 30 July 2021, we enrolled 1538 patients from 11 Dutch hospitals undergoing transrectal PB. After rectal swab collection, patients were randomized 1:1 to receive empirical prophylaxis with oral ciprofloxacin (control group [CG]) or culture-based prophylaxis (intervention group [IG]). Primary outcome was any infectious complication within 7 days after biopsy. Secondary outcomes were infectious complications within 30 days, and bacteremia and bacteriuria within 7 and 30 days postbiopsy. For primary outcome analysis, the χ2 test stratified for hospitals was used. Trial registration number: NCT03228108.
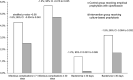
Results: Data from 1288 patients (83.7%) were available for analysis (CG, 652; IG, 636). Infection rates within 7 days postbiopsy were 4.3% (n = 28) (CG) and 2.5% (n = 16) (IG) (P value = .08; reduction: -1.8%; 95% confidence interval, -.004 to .040). Ciprofloxacin-resistant bacteria were detected in 15.2% (n = 1288). In the CG, the presence of ciprofloxacin-resistant rectal flora resulted in a 6.2-fold higher risk of early postbiopsy infection.
Conclusions: Our study supports the use of culture-based prophylaxis to reduce infectious complications after transrectal PB. Despite adequate prophylaxis, postbiopsy infections can still occur. Therefore, culture-based prophylaxis must be weighed against other strategies that could reduce postbiopsy infections. Clinical Trials Registration. NCT03228108.
Keywords: culture-based antibiotic prophylaxis; empirical antibiotic prophylaxis; infectious complications; transrectal prostate biopsy.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. D. M. S. reports grants or contracts from Besins and Astellas; payment for expert testimony from Bayer, Janssen, and MSD; a leadership or fiduciary role with the Dutch Urological Association; and receipt of equipment, materials, drugs, medical writing, gifts, or other services from Samantree and Besins. The other authors reported no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Figures


Comment in
-
Tailored prophylaxis for prostate biopsy.Nat Rev Urol. 2023 Feb;20(2):63. doi: 10.1038/s41585-022-00701-2. Nat Rev Urol. 2023. PMID: 36481923 No abstract available.
References
-
- Loeb S, van den Heuvel S, Zhu X, Bangma CH, Schroder FH, Roobol MJ. Infectious complications and hospital admissions after prostate biopsy in a European randomized trial. Eur Urol 2012; 61:1110–4. - PubMed
-
- Verburgh HA, Neeling de H. Nethmap 2003: Consumption of antimicrobial agents and antimicrobial resistance among medically important bacteria in The Netherlands. RIVM Bilthoven, 2003. https://swab.nl/nl/nethmap
-
- Greeff de SC, Mouton JW. Nethmap 2017: consumption of antimicrobial agents and antimicrobial resistance among medically important bacteria in The Netherlands. RIVM Bilthoven, 2017. https://swab.nl/nl/nethmap
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

