Modulation of cortical beta oscillations influences motor vigor: A rhythmic TMS-EEG study
- PMID: 36419365
- PMCID: PMC9875933
- DOI: 10.1002/hbm.26149
Modulation of cortical beta oscillations influences motor vigor: A rhythmic TMS-EEG study
Abstract
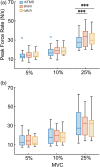
Previous electro- or magnetoencephalography (Electro/Magneto EncephaloGraphic; E/MEG) studies using a correlative approach have shown that β (13-30 Hz) oscillations emerging in the primary motor cortex (M1) are implicated in regulating motor response vigor and associated with an anti-kinetic role, that is, slowness of movement. However, the functional role of M1 β oscillations in regulation of motor responses remains unclear. To address this gap, we combined EEG with rhythmic TMS (rhTMS) delivered to M1 at the β (20 Hz) frequency shortly before subjects performed an isometric ramp-and-hold finger force production task at three force levels. rhTMS is a novel approach that can modulate rhythmic patterns of neural activity. β-rhTMS over M1 induced a modulation of neural oscillations to β frequency in the sensorimotor area and reduced peak force rate during the ramp-up period relative to sham and catch trials. Interestingly, this rhTMS effect occurred only in the large force production condition. To distinguish whether the effects of rhTMS on EEG and behavior stemmed from phase-resetting by each magnetic pulse or neural entrainment by the periodicity of rhTMS, we performed a control experiment using arrhythmic TMS (arTMS). arTMS did not induce changes in EEG oscillations nor peak force rate during the rump-up period. Our results provide novel evidence that β neural oscillations emerging the sensorimotor area influence the regulation of motor response vigor. Furthermore, our findings further demonstrate that rhTMS is a promising tool for tuning neural oscillations to the target frequency.
Keywords: cortical oscillations; motor control; primary motor cortex.
© 2022 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Figures





References
-
- Albouy, P. , Weiss, A. , Baillet, S. , & Zatorre, R. J. (2017). Selective entrainment of theta oscillations in the dorsal stream causally enhances auditory working memory performance. Neuron, 94, 193–206. - PubMed
-
- Androulidakis, A. G. , Doyle, L. M. F. , Gilbertson, T. P. , & Brown, P. (2006). Corrective movements in response to displacements in visual feedback are more effective during periods of 13–35 Hz oscillatory synchrony in the human corticospinal system. The European Journal of Neuroscience, 24, 3299–3304. - PubMed
-
- Androulidakis, A. G. , Doyle, L. M. F. , Yarrow, K. , Litvak, V. , Gilbertson, T. P. , & Brown, P. (2007). Anticipatory changes in beta synchrony in the human corticospinal system and associated improvements in task performance. The European Journal of Neuroscience, 25, 3758–3765. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

