Brain transplantation of genetically corrected Sanfilippo type B neural stem cells induces partial cross-correction of the disease
- PMID: 36419468
- PMCID: PMC9672419
- DOI: 10.1016/j.omtm.2022.10.013
Brain transplantation of genetically corrected Sanfilippo type B neural stem cells induces partial cross-correction of the disease
Abstract
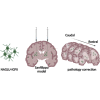
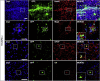
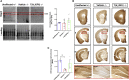
Sanfilippo syndrome type B (mucopolysaccharidosis type IIIB) is a recessive genetic disorder that severely affects the brain due to a deficiency in the enzyme α-N-acetylglucosaminidase (NAGLU), leading to intra-lysosomal accumulation of partially degraded heparan sulfate. There are no effective treatments for this disorder. In this project, we carried out an ex vivo correction of neural stem cells derived from Naglu -/- mice (iNSCs) induced pluripotent stem cells (iPSC) using a modified enzyme in which human NAGLU is fused to an insulin-like growth factor II receptor binding peptide in order to improve enzyme uptake. After brain transplantation of corrected iNSCs into Naglu -/- mice and long-term evaluation of their impact, we successfully detected NAGLU-IGFII activity in all transplanted animals. We found decreased lysosomal accumulation and reduced astrocytosis and microglial activation throughout transplanted brains. We also identified a novel neuropathological phenotype in untreated Naglu -/- brains with decreased levels of the neuronal marker Map2 and accumulation of synaptophysin-positive aggregates. Upon transplantation, we restored levels of Map2 expression and significantly reduced formation of synaptophysin-positive aggregates. Our findings suggest that genetically engineered iNSCs can be used to effectively deliver the missing enzyme to the brain and treat Sanfilippo type B-associated neuropathology.
Keywords: LSD; MPS; Sanfilippo type B; cell therapy; neural progenitor cells.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Fraldi A., Annunziata F., Lombardi A., Kaiser H.J., Medina D.L., Spampanato C., Fedele A.O., Polishchuk R., Sorrentino N.C., Simons K., et al. Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders. EMBO J. 2010;29:3607–3620. doi: 10.1038/emboj.2010.237. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

