MicroRNA panels as diagnostic biomarkers for colorectal cancer: A systematic review and meta-analysis
- PMID: 36419785
- PMCID: PMC9676370
- DOI: 10.3389/fmed.2022.915226
MicroRNA panels as diagnostic biomarkers for colorectal cancer: A systematic review and meta-analysis
Abstract
Background: Circulating microRNAs (miRNA) have emerged as promising diagnostic biomarkers for several diseases, including cancer. However, the diagnostic accuracy of miRNA panels in colorectal cancer (CRC) remains inconsistent and there is still lack of meta-analyses to determine whether miRNA panels can serve as robust biomarkers for CRC diagnosis.
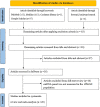
Methods: This study performed a systematic review and meta-analysis to evaluate the clinical utility of miRNA panels as potential biomarkers for the diagnosis of CRC. The investigation systematically searched PubMed, Medline, Web of Science, Cochrane Library, and Google Scholar (21-year span, between 2000 and 2021) to retrieve articles reporting the diagnostic role of miRNA panels in detecting CRC. Diagnostic meta-analysis of miRNA panels used diverse evaluation indicators, including sensitivity, specificity, Positive Likelihood Ratio (PLR), Negative Likelihood Ratio (NLR), Diagnostic Odds Ratio (DOR), and the area under the curve (AUC) values.
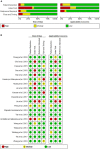
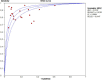
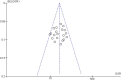
Results: Among the 313 articles identified, 20 studies met the inclusion criteria. The pooled estimates of miRNA panels for the diagnosis of CRC were 0.85 (95% CI: 0.84-0.86), 0.79 (95% CI: 0.78-0.80), 4.06 (95% CI: 3.89-4.23), 0.20 (95% CI: 0.19-0.20), 22.50 (95% CI: 20.81-24.32) for sensitivity, specificity, PLR, NLR, and DOR, respectively. Moreover, the summary receiver operating characteristics (SROC) curve revealed an AUC value of 0.915 (95% CI: 0.914-0.916), suggesting an outstanding diagnostic accuracy for overall miRNA panels. Subgroup and meta-regression analyses demonstrated that miRNA panels have the highest diagnostic accuracy within serum samples, rather than in other sample-types - with a sensitivity, specificity, PLR, NLR, DOR, and AUC of 0.87, 0.86, 7.33, 0.13, 55.29, and 0.943, respectively. Sensitivity analysis revealed that DOR values did not differ markedly, which indicates that the meta-analysis had strong reliability. Furthermore, this study demonstrated no proof of publication bias for DOR values analyzed using Egger's regression test (P > 0.05) and funnel plot. Interestingly, miR-15b, miR-21 and miR-31 presented the best diagnostic accuracy values for CRC with sensitivity, specificity, PLR, NLR, DOR, and AUC values of 0.95, 0.94, 17.19, 0.05, 324.81, and 0.948, respectively.
Conclusion: This study's findings indicated that miRNA panels, particularly serum-derived miRNA panels, can serve as powerful and promising biomarkers for early CRC screening.
Systematic review registration: [www.crd.york.ac.uk/prospero], identifier [CRD42021268172].
Keywords: biomarker; colorectal cancer; diagnostic; miRNA; panels.
Copyright © 2022 Sur, Advani and Braithwaite.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Bakkers C, Simkens GAAM, De Hingh IHJT. Systemic therapy in addition to cytoreduction and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastases: recent insights from clinical studies and translational research. J Gastrointest Oncol. (2021) 12:S206–13. 10.21037/jgo-20-133 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

