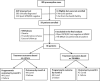
Systematic assessment of clinical and bacteriological markers for tuberculosis reveals discordance and inaccuracy of symptom-based diagnosis for treatment response monitoring
- PMID: 36419786
- PMCID: PMC9677322
- DOI: 10.3389/fmed.2022.992451
Systematic assessment of clinical and bacteriological markers for tuberculosis reveals discordance and inaccuracy of symptom-based diagnosis for treatment response monitoring
Abstract
Background: Clinical symptoms are the benchmark of tuberculosis (TB) diagnosis and monitoring of treatment response but are not clear how they relate to TB bacteriology, particularly the novel tuberculosis-molecular bacterial load assay (TB-MBLA).
Methods: Presumptive cases were bacteriologically confirmed for TB and assessed for symptoms and bacteriological resolution using smear microscopy (SM), culture, and TB-MBLA over 6-month treatment course. Kaplan-Meier and Kappa statistics were used to test the relationship between symptoms and bacteriological positivity.
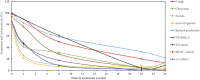
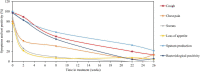
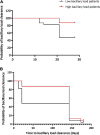
Results: A cohort of 46 bacteriologically confirmed TB cases were analyzed for treatment response over a 6-month treatment course. Pre-treatment symptoms and bacteriological positivity concurred in over 70% of the cases. This agreement was lost in over 50% of cases whose chest pain, night sweat, and loss of appetite had resolved by week 2 of treatment. Cough resolved at a 3.2% rate weekly and was 0.3% slower than the combined bacteriological (average of MGIT and TB-MBLA positivity) resolution rate, 3.5% per week. A decrease in TB-MBLA positivity reflected a fall in bacillary load, 5.7 ± 1.3- at baseline to 0.30 ± 1.0- log10 eCFU/ml at month 6, and closer to cough resolution than other bacteriological measures, accounting for the only one bacteriologically positive case out of seven still coughing at month 6. Low baseline bacillary load patients were more likely to be bacteriologically negative, HR 5.6, p = 0.003 and HR 3.2, p = 0.014 by months 2 and 6 of treatment, respectively.
Conclusion: The probability of clinical symptoms reflecting bacteriological positivity weakens as the patient progresses on anti-TB therapy, making the symptom-based diagnosis a less reliable marker of treatment response.
Keywords: TB symptoms; TB-MBLA; bacteriological tests; diagnosis; monitoring.
Copyright © 2022 Mtafya, Sabi, John, Sichone, Olomi, Gillespie, Ntinginya and Sabiiti.
Conflict of interest statement
Life Arc, which is developing TB-MBLA for clinical use, receives pro bono advice from SG and WS. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Maclean E, Mckenna L, Ruhwald M. Pipeline Report 2021. New York, NY: Treatment Action Group; (2021).
-
- World Health Organization. Global Tuberculosis Report. Geneva: World Health Organization; (2021).
-
- Tiberi S, du Plessis N, Walzl G, Vjecha MJ, Rao M, Ntoumi F, et al. Tuberculosis: progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect Dis. (2018) 18:e183–98. - PubMed
LinkOut - more resources
Full Text Sources

