Usefulness of malignant pleural effusion for early cytological diagnosis of mesothelioma in situ: A case report
- PMID: 36420072
- PMCID: PMC9641808
- DOI: 10.3892/ol.2022.13560
Usefulness of malignant pleural effusion for early cytological diagnosis of mesothelioma in situ: A case report
Abstract
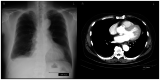
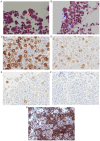
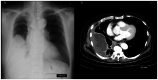
Mesothelioma in situ (MIS) is defined as a preinvasive mesothelioma that forms a single layer of mild atypical mesothelial cells lining on the serosa surface of pleura. The atypical mesothelial cells present loss of BRCA-1 associated protein-1 (BAP-1) and/or methylthioadenosine phosphorylase as examined by immunohistochemistry (IHC) and/or homozygous deletion of cyclin-dependent kinase inhibitor 2A/p16 as examined by fluorescence in situ hybridization. It is difficult to diagnose because of the unremarkable clinical findings except for pleural effusion. The present report describes a case in which MIS was diagnosed at the time of sampling due to the presence of clearly malignant mesothelial cells in the pleural fluid. In 2016, a 74-year-old man with a history of past exposure to asbestos was admitted to Ibaraki Higashi National Hospital (Tokai-mura, Japan) with dyspnea. Chest CT indicated only right pleural effusion. Malignant mesothelial cells were suspected in a cell block made using pleural effusion; therefore, right pleural biopsy was performed. Pathologically, there was proliferation of mesothelial cells with mild atypia that formed a single-flat layer on the pleural surface; however, there was no invasion. Furthermore, IHC revealed loss of BAP-1 in cells from the biopsied pleura and pleural effusion. MIS was suspected at the time; however, the patient arbitrarily quit his medical check-ups. After 44 months, the patient was readmitted to our hospital complaining of dyspnea. CT indicated a large right pleural mass. A specimen of the mass obtained via CT-guided needle biopsy revealed malignant mesothelioma. The patient continued to deteriorate and eventually died. This case indicated that pleural effusion could be used to demonstrate overtly malignant mesothelial cells and diagnose MIS at the time of sampling. To the best of our knowledge, this is first report of MIS with overtly malignant mesothelial cells in pleural effusion. Pleural effusion may serve an important role in MIS diagnosis.
Keywords: BAP-1; atypical cells; cell block; first obtained pleural fluid; malignant mesothelial cells.
Copyright: © Yabuuchi et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Malignant mesothelioma in situ diagnosed by methylthioadenosine phosphorylase loss and homozygous deletion of CDKN2A: a case report.Virchows Arch. 2020 Mar;476(3):469-473. doi: 10.1007/s00428-019-02674-x. Epub 2019 Oct 30. Virchows Arch. 2020. PMID: 31667596
-
Usefulness of methylthioadenosine phosphorylase and BRCA-associated protein 1 immunohistochemistry in the diagnosis of malignant mesothelioma in effusion cytology specimens.Cancer Cytopathol. 2020 Feb;128(2):126-132. doi: 10.1002/cncy.22221. Epub 2019 Dec 10. Cancer Cytopathol. 2020. PMID: 31821740
-
Sarcomatoid mesothelioma diagnosed in a patient with mesothelioma in situ: a case report on morphologic differences after 9-month interval with details analysis of cytology in early-stage mesothelioma.Diagn Pathol. 2023 Nov 28;18(1):126. doi: 10.1186/s13000-023-01416-7. Diagn Pathol. 2023. PMID: 38017544 Free PMC article.
-
Diagnostic mesothelioma biomarkers in effusion cytology.Cancer Cytopathol. 2021 Jul;129(7):506-516. doi: 10.1002/cncy.22398. Epub 2021 Jan 19. Cancer Cytopathol. 2021. PMID: 33465294 Review.
-
The cytological features of effusions with mesothelioma in situ: A report of 9 cases.Diagn Cytopathol. 2023 Jun;51(6):374-388. doi: 10.1002/dc.25129. Epub 2023 Mar 21. Diagn Cytopathol. 2023. PMID: 36942732 Review.
Cited by
-
Malignant Mesothelioma: Overcoming Diagnostic Hurdles.Cureus. 2024 Sep 5;16(9):e68718. doi: 10.7759/cureus.68718. eCollection 2024 Sep. Cureus. 2024. PMID: 39371847 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
