PROTACs in gastrointestinal cancers
- PMID: 36420306
- PMCID: PMC9676279
- DOI: 10.1016/j.omto.2022.10.012
PROTACs in gastrointestinal cancers
Abstract
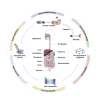
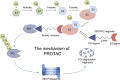
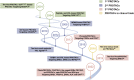
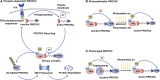
Proteolysis targeting chimera (PROTAC) presents a powerful strategy for targeted protein degradation (TPD). The heterobifunctional PROTAC molecule consists of an E3 ligase ligand covalently linked to a protein of interest (POI) via a linker. PROTAC can induce ubiquitinated proteasomal degradation of proteins by hijacking the ubiquitin-proteasome degradation system (UPS). This technique has the advantages of broad targeting profile, good cell permeability, tissue specificity, high selectivity, oral bioavailability, and controllability. To date, a growing number of PROTACs targeting gastrointestinal cancers have been successfully developed, and, in many cases, their POIs have been validated as clinical drug targets. To the best of our knowledge, 15 PROTACs against various targets are currently tested in clinical trials, and many more are likely to be added in the near future. Therefore, this paper details the mechanism, research progress, and application in clinical trials of PROTACs, and summarizes the research achievements related to PROTACs in gastrointestinal cancers. Finally, we discuss the advantages and potential challenges of PROTAC for cancer treatment.
Keywords: E3 ligases; PROTACs; cancer treatment; gastrointestinal cancers; targeted protein degradation.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Recent Advances in PROTACs for Drug Targeted Protein Research.Int J Mol Sci. 2022 Sep 7;23(18):10328. doi: 10.3390/ijms231810328. Int J Mol Sci. 2022. PMID: 36142231 Free PMC article. Review.
-
Advances and perspectives of proteolysis targeting chimeras (PROTACs) in drug discovery.Bioorg Chem. 2022 Aug;125:105848. doi: 10.1016/j.bioorg.2022.105848. Epub 2022 May 5. Bioorg Chem. 2022. PMID: 35533582 Review.
-
Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy.J Hematol Oncol. 2020 May 13;13(1):50. doi: 10.1186/s13045-020-00885-3. J Hematol Oncol. 2020. PMID: 32404196 Free PMC article. Review.
-
PROteolysis TArgeting Chimeras (PROTACs) as emerging anticancer therapeutics.Oncogene. 2020 Jun;39(26):4909-4924. doi: 10.1038/s41388-020-1336-y. Epub 2020 May 31. Oncogene. 2020. PMID: 32475992 Free PMC article. Review.
-
Proteolysis-targeting chimeras (PROTACs) in cancer therapy.Mol Cancer. 2022 Apr 11;21(1):99. doi: 10.1186/s12943-021-01434-3. Mol Cancer. 2022. PMID: 35410300 Free PMC article. Review.
Cited by
-
PROTACs: A novel strategy for cancer drug discovery and development.MedComm (2020). 2023 May 29;4(3):e290. doi: 10.1002/mco2.290. eCollection 2023 Jun. MedComm (2020). 2023. PMID: 37261210 Free PMC article. Review.
-
Small Molecule Inhibitors as Therapeutic Agents Targeting Oncogenic Fusion Proteins: Current Status and Clinical.Molecules. 2023 Jun 9;28(12):4672. doi: 10.3390/molecules28124672. Molecules. 2023. PMID: 37375228 Free PMC article. Review.
-
Advances in molecular targeted therapies to increase efficacy of (chemo)radiation therapy.Strahlenther Onkol. 2023 Dec;199(12):1091-1109. doi: 10.1007/s00066-023-02064-y. Epub 2023 Apr 11. Strahlenther Onkol. 2023. PMID: 37041372 Free PMC article. Review.
-
Cancer-Specific Delivery of Proteolysis-Targeting Chimeras (PROTACs) and Their Application to Cancer Immunotherapy.Pharmaceutics. 2023 Jan 26;15(2):411. doi: 10.3390/pharmaceutics15020411. Pharmaceutics. 2023. PMID: 36839734 Free PMC article. Review.
-
Digestive cancers: mechanisms, therapeutics and management.Signal Transduct Target Ther. 2025 Jan 15;10(1):24. doi: 10.1038/s41392-024-02097-4. Signal Transduct Target Ther. 2025. PMID: 39809756 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

