Nucleic acid vaccination strategies for ovarian cancer
- PMID: 36420446
- PMCID: PMC9677957
- DOI: 10.3389/fbioe.2022.953887
Nucleic acid vaccination strategies for ovarian cancer
Abstract
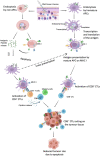
High grade serous carcinoma (HGSC) is one of the most lethal ovarian cancers that is characterised by asymptomatic tumour growth, insufficient knowledge of malignant cell origin and sub-optimal detection. HGSC has been recently shown to originate in the fallopian tube and not in the ovaries. Conventional treatments such as chemotherapy and surgery depend upon the stage of the disease and have resulted in higher rates of relapse. Hence, there is a need for alternative treatments. Differential antigen expression levels have been utilised for early detection of the cancer and could be employed in vaccination strategies using nucleic acids. In this review the different vaccination strategies in Ovarian cancer are discussed and reviewed. Nucleic acid vaccination strategies have been proven to produce a higher CD8+ CTL response alongside CD4+ T-cell response when compared to other vaccination strategies and thus provide a good arena for antitumour immune therapy. DNA and mRNA need to be delivered into the intracellular matrix. To overcome ineffective naked delivery of the nucleic acid cargo, a suitable delivery system is required. This review also considers the suitability of cell penetrating peptides as a tool for nucleic acid vaccine delivery in ovarian cancer.
Keywords: DNA; high grade serous carcinoma; mRNA; nucleic acid vaccines; ovarian cancer; tumour antigens.
Copyright © 2022 Saha, Bojdo, Dunne, Duary, Buckley and McCarthy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



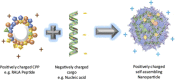
References
-
- Antoniou A., Pharoah P. D., Narod S., Risch H. A., Eyfjord J. E., Hopper J. L., et al. (2003). Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am. J. Hum. Genet. 72 (5), 1117–1130. 10.1086/375033 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

