Dual-Color Photoconvertible Fluorescent Probes Based on Directed Photooxidation Induced Conversion for Bioimaging
- PMID: 36420823
- PMCID: PMC10107923
- DOI: 10.1002/anie.202215085
Dual-Color Photoconvertible Fluorescent Probes Based on Directed Photooxidation Induced Conversion for Bioimaging
Abstract
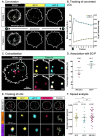
We herein present a new concept to produce dual-color photoconvertible probes based on a mechanism called Directed Photooxidation Induced Conversion (DPIC). As a support of this mechanism, styryl-coumarins (SCs) bearing Aromatic Singlet Oxygen Reactive Moieties (ASORMs) like furan and pyrrole have been synthesized. SCs are bright fluorophores, which undergo a hypsochromic conversion upon visible light irradiation due to directed photooxidation of the ASORM that leads to the disruption of conjugation. SC-P, a yellow emitting probe bearing a pyrrole moiety, converts to a stable blue emitting coumarin with a 68 nm shift allowing the photoconversion and tracking of lipid droplet in live cells. This new approach might pave the way to a new generation of photoconvertible dyes for advanced bioimaging applications.
Keywords: Fluorescent Probes; Lipid Droplets; Photoconversion; Photooxidation; Photoswitching.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Patterson G. H., Lippincott-Schwartz J., Science 2002, 297, 1873–1877. - PubMed
-
- Lukyanov K. A., Chudakov D. M., Lukyanov S., Verkhusha V. V., Nat. Rev. Mol. Cell Biol. 2005, 6, 885–890. - PubMed
-
- Lavis L. D., Biochemistry 2017, 56, 5165–5170. - PubMed
-
- Trinh N., Jolliffe K. A., New E. J., Angew. Chem. Int. Ed. 2020, 59, 20290–20301; - PubMed
- Angew. Chem. 2020, 132, 20466–20479.
-
- Li H., Kim D., Yao Q., Ge H., Chung J., Fan J., Wang J., Peng X., Yoon J., Angew. Chem. 2021, 133, 17408–17429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

