Treatment of enteric fever (typhoid and paratyphoid fever) with cephalosporins
- PMID: 36420914
- PMCID: PMC9686137
- DOI: 10.1002/14651858.CD010452.pub2
Treatment of enteric fever (typhoid and paratyphoid fever) with cephalosporins
Abstract
Background: Typhoid and paratyphoid (enteric fever) are febrile bacterial illnesses common in many low- and middle-income countries. The World Health Organization (WHO) currently recommends treatment with azithromycin, ciprofloxacin, or ceftriaxone due to widespread resistance to older, first-line antimicrobials. Resistance patterns vary in different locations and are changing over time. Fluoroquinolone resistance in South Asia often precludes the use of ciprofloxacin. Extensively drug-resistant strains of enteric fever have emerged in Pakistan. In some areas of the world, susceptibility to old first-line antimicrobials, such as chloramphenicol, has re-appeared. A Cochrane Review of the use of fluoroquinolones and azithromycin in the treatment of enteric fever has previously been undertaken, but the use of cephalosporins has not been systematically investigated and the optimal choice of drug and duration of treatment are uncertain.
Objectives: To evaluate the effectiveness of cephalosporins for treating enteric fever in children and adults compared to other antimicrobials.
Search methods: We searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL, MEDLINE, Embase, LILACS, the WHO ICTRP and ClinicalTrials.gov up to 24 November 2021. We also searched reference lists of included trials, contacted researchers working in the field, and contacted relevant organizations.
Selection criteria: We included randomized controlled trials (RCTs) in adults and children with enteric fever that compared a cephalosporin to another antimicrobial, a different cephalosporin, or a different treatment duration of the intervention cephalosporin. Enteric fever was diagnosed on the basis of blood culture, bone marrow culture, or molecular tests.
Data collection and analysis: We used standard Cochrane methods. Our primary outcomes were clinical failure, microbiological failure and relapse. Our secondary outcomes were time to defervescence, duration of hospital admission, convalescent faecal carriage, and adverse effects. We used the GRADE approach to assess certainty of evidence for each outcome.
Main results: We included 27 RCTs with 2231 total participants published between 1986 and 2016 across Africa, Asia, Europe, the Middle East and the Caribbean, with comparisons between cephalosporins and other antimicrobials used for the treatment of enteric fever in children and adults. The main comparisons are between antimicrobials in most common clinical use, namely cephalosporins compared to a fluoroquinolone and cephalosporins compared to azithromycin. Cephalosporin (cefixime) versus fluoroquinolones Clinical failure, microbiological failure and relapse may be increased in patients treated with cefixime compared to fluoroquinolones in three small trials published over 14 years ago: clinical failure (risk ratio (RR) 13.39, 95% confidence interval (CI) 3.24 to 55.39; 2 trials, 240 participants; low-certainty evidence); microbiological failure (RR 4.07, 95% CI 0.46 to 36.41; 2 trials, 240 participants; low-certainty evidence); relapse (RR 4.45, 95% CI 1.11 to 17.84; 2 trials, 220 participants; low-certainty evidence). Time to defervescence in participants treated with cefixime may be longer compared to participants treated with fluoroquinolones (mean difference (MD) 1.74 days, 95% CI 0.50 to 2.98, 3 trials, 425 participants; low-certainty evidence). Cephalosporin (ceftriaxone) versus azithromycin Ceftriaxone may result in a decrease in clinical failure compared to azithromycin, and it is unclear whether ceftriaxone has an effect on microbiological failure compared to azithromycin in two small trials published over 18 years ago and in one more recent trial, all conducted in participants under 18 years of age: clinical failure (RR 0.42, 95% CI 0.11 to 1.57; 3 trials, 196 participants; low-certainty evidence); microbiological failure (RR 1.95, 95% CI 0.36 to 10.64, 3 trials, 196 participants; very low-certainty evidence). It is unclear whether ceftriaxone increases or decreases relapse compared to azithromycin (RR 10.05, 95% CI 1.93 to 52.38; 3 trials, 185 participants; very low-certainty evidence). Time to defervescence in participants treated with ceftriaxone may be shorter compared to participants treated with azithromycin (mean difference of -0.52 days, 95% CI -0.91 to -0.12; 3 trials, 196 participants; low-certainty evidence). Cephalosporin (ceftriaxone) versus fluoroquinolones It is unclear whether ceftriaxone has an effect on clinical failure, microbiological failure, relapse, and time to defervescence compared to fluoroquinolones in three trials published over 28 years ago and two more recent trials: clinical failure (RR 3.77, 95% CI 0.72 to 19.81; 4 trials, 359 participants; very low-certainty evidence); microbiological failure (RR 1.65, 95% CI 0.40 to 6.83; 3 trials, 316 participants; very low-certainty evidence); relapse (RR 0.95, 95% CI 0.31 to 2.92; 3 trials, 297 participants; very low-certainty evidence) and time to defervescence (MD 2.73 days, 95% CI -0.37 to 5.84; 3 trials, 285 participants; very low-certainty evidence). It is unclear whether ceftriaxone decreases convalescent faecal carriage compared to the fluoroquinolone gatifloxacin (RR 0.18, 95% CI 0.01 to 3.72; 1 trial, 73 participants; very low-certainty evidence) and length of hospital stay may be longer in participants treated with ceftriaxone compared to participants treated with the fluoroquinolone ofloxacin (mean of 12 days (range 7 to 23 days) in the ceftriaxone group compared to a mean of 9 days (range 6 to 13 days) in the ofloxacin group; 1 trial, 47 participants; low-certainty evidence).
Authors' conclusions: Based on very low- to low-certainty evidence, ceftriaxone is an effective treatment for adults and children with enteric fever, with few adverse effects. Trials suggest that there may be no difference in the performance of ceftriaxone compared with azithromycin, fluoroquinolones, or chloramphenicol. Cefixime can also be used for treatment of enteric fever but may not perform as well as fluoroquinolones. We are unable to draw firm general conclusions on comparative contemporary effectiveness given that most trials were small and conducted over 20 years previously. Clinicians need to take into account current, local resistance patterns in addition to route of administration when choosing an antimicrobial.
Copyright © 2022 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
RK is a Cochrane Infectious Diseases Group Research Associate, and was not involved in the editorial process. She has no known conflicts of interest.
NS has no known conflicts of interest.
DE has received grants from the NIHR, Gilead Sciences, and the Robertson Foundation, and has no known conflicts of interest.
TD is a previous contributor to WHO Guidance for the Surveillance of Vaccine Preventable Diseases (typhoid), and has no known conflicts of interest.
BB is a co‐author on two trials included in this review (Arjyal 2016; Pandit 2007).
CP is a co‐author on the following trial included in this review (Cao 1999).
Figures
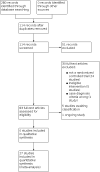







































Update of
References
References to studies included in this review
Acharya 1995 {published data only}
-
- Acharya G, Butler T, Ho M, Sharma PR, Tiwari M, Adhikari RK, et al. Treatment of typhoid fever: randomized trial of a three-day course of ceftriaxone versus a fourteen-day course of chloramphenicol. American Journal of Tropical Medicine and Hygiene 1995;52(2):162-5. - PubMed
Arjyal 2016 {published data only}
Bhutta 1994 {published data only}
-
- Bhutta ZA, Khan IA, Molla AM. Therapy of multidrug-resistant typhoid fever with oral cefixime vs. intravenous ceftriaxone. Pediatric Infectious Disease Journal 1994;13(11):990-4. - PubMed
-
- Bhutta ZA, Mansoorali N, Hussain R. Plasma cytokines in paediatric typhoidal salmonellosis: correlation with clinical course and outcome. Journal of Infection 1997;35(3):253-6. - PubMed
Bhutta 2000 {published data only}
Butler 1993 {published data only}
Cao 1999 {published data only}
-
- Cao XT, Kneen R, Nguyen TA, Truong DL, White NJ, Parry CM. A comparative study of ofloxacin and cefixime for treatment of typhoid fever in children. The Dong Nai Pediatric Center Typhoid Study Group. Paediatric Infectious Disease Journal 1999;18(3):245-8. - PubMed
Frenck 2000 {published data only}
-
- Frenck RW Jr, Nakhla I, Sultan Y, Bassily SB, Girgis YF, David J, et al. Azithromycin versus ceftriaxone for the treatment of uncomplicated typhoid fever in children. Clinical Infectious Diseases 2000;31(5):1134-8. - PubMed
Frenck 2004 {published data only}
-
- Frenck RW Jr, Mansour A, Nakhla I, Sultan Y, Putnam S, Wierzba T, et al. Short-course azithromycin for the treatment of uncomplicated typhoid fever in children and adolescents. Clinical Infectious Diseases 2004;38(7):951-7. - PubMed
Girgis 1990 {published data only}
-
- Girgis NI, Kilpatrick ME, Farid Z, Mikhail IA, Bishay E. Ceftriaxone versus chloramphenicol in the treatment of enteric fever. Drugs under Experimental and Clinical Research 1990;16(12):607-9. - PubMed
Girgis 1995 {published data only}
-
- Girgis NI, Sultan Y, Hammad O, Farid Z. Comparison of the efficacy, safety and cost of cefixime, ceftriaxone and aztreonam in the treatment of multidrug-resistant Salmonella typhi septicemia in children. Pediatric Infectious Disease Journal 1995;14(7):603-5. - PubMed
Islam 1988 {published data only}
-
- Islam A, Butler T, Nath SK, Alam NH, Stoeckel K, Houser HB, et al. Randomized treatment of patients with typhoid fever by using ceftriaxone or chloramphenicol. Journal of Infectious Diseases 1988;158(4):742-7. - PubMed
Islam 1993 {published data only}
Kumar 2007 {published data only}
-
- Kumar R, Gupta N, Shalini. Multidrug-resistant typhoid fever. Indian Journal of Pediatrics 2007;74(1):39-42. - PubMed
Lasserre 1991 {published data only}
-
- Lasserre R, Sangalang RP, Santiago L. Three-day treatment of typhoid fever with two different doses of ceftriaxone, compared to 14-day therapy with chloramphenicol: a randomized trial. Journal of Antimicrobial Chemotherapy 1991;28(5):765-72. - PubMed
Memon 1997 {published data only}
-
- Memon IA, Billoo AG, Memon HI. Cefixime: an oral option for the treatment of multidrug-resistant enteric fever in children. Southern Medical Journal 1997;90(12):1204-7. - PubMed
Moosa 1989 {published data only}
-
- Moosa A, Rubidge CJ. Once daily ceftriaxone vs. chloramphenicol for treatment of typhoid fever in children. Pediatric Infectious Disease Journal 1989;8(10):696-9. - PubMed
Morelli 1988 {published data only}
-
- Morelli G, Guerriero M, Cristiano P, Galderisi P, Postiglione A, Paradisi F. Cefoperazone compared with chloramphenicol in the treatment of typhoid fever. Chemotherapy 1988;34(1):71-6. - PubMed
Nair 2017 {published data only}
-
- Nair BT, Simalti AK, Sharma S. Study comparing ceftriaxone with azithromycin for the treatment of uncomplicated typhoid fever in children of India. Annals of Tropical Medicine and Public Health 2017;10(1):205-10.
Pandit 2007 {published and unpublished data}
Pape 1986 {published data only}
-
- Pape JW, Gerdes H, Oriol L, Johnson WD Jr. Typhoid fever: successful therapy with cefoperazone. Journal of Infectious Diseases 1986;153(2):272-6. - PubMed
Rabbani 1998 {published data only}
-
- Malik MS, Iqbal I, Rabbani W. A comparative study of cefixime and chloramphenicol in children with typhoid fever. Journal of Pakistan Medical Association 1998;48(4):106-7. - PubMed
-
- Rabbani MW, Iqbal I, Malik MS. A comparative study of cefixime and chloramphenicol in children with typhoid fever. Journal of the Pakistan Medical Association 1998;48(6):163-4. - PubMed
Rizvi 2007 {published data only}
-
- Rizvi Q. Effectiveness of anti-typhoid drugs currently used in Pakistan. Pakistan Journal of Surgery 2007;23(1):57-64.
Shakur 2007 {published data only}
-
- Shakur MS, Arzuman SA, Hossain J, Mehdi H, Ahmed M. Cefpodoxime proxetil compared with cefixime for treatment of typhoid fever in children. Indian Pediatrics 2007;44(11):838-41. - PubMed
Smith 1994 {published data only}
Tatli 2003 {published data only}
-
- Tatli MM, Aktas G, Kosecik M, Yilmaz A. Treatment of typhoid fever in children with a flexible-duration of ceftriaxone, compared with 14-day treatment with chloramphenicol. International Journal of Antimicrobial Agents 2003;21(4):350-3. - PubMed
Tran 1994 {published data only}
-
- Tran TH, Nguyen MD, Huynh DH, Nguyen TT, To SD, Le TP, et al. A randomized comparative study of fleroxacin and ceftriaxone in enteric fever. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88(4):464-5. - PubMed
Wallace 1993 {published data only}
-
- Wallace MR, Yousif AA, Mahroos GA, Mapes T, Threlfall EJ, Rowe B, et al. Ciprofloxacin versus ceftriaxone in the treatment of multiresistant typhoid fever. European Journal of Clinical Microbiology & Infectious Diseases 1993;12(12):907-10. - PubMed
References to studies excluded from this review
Arjyal 2011 {published data only}
Begue 1998 {published data only}
-
- Begue P, Astruc J, Francois P, Floret D. Comparison of ceftriaxone and cefotaxime in severe pediatric bacterial infection: a multicentric study. Médecine et Maladies Infectieuses 1998;27:300-6.
Begum 2014 {published data only}
-
- Begum B, Haque MA, Ahmed MS, Islam MN, Ahsan MM, Khan AH, et al. Comparison between azithromycin and cefixime in the treatment of typhoid fever in children. Mymensingh Medical Journal 2014;23(3):441-8. - PubMed
De Carvalho 1982 {published data only}
Ebright 1983 {published data only}
-
- Ebright JR, Franson TR, Moore EC. Comparative trial of ceftizoxime and cefamandole in therapy of bacterial pneumonia. Current Therapeutic Research 1983;33(1):39-45.
Farid 1987 {published data only}
-
- Farid Z, Girgis N, Abu el Ella A. Successful treatment of typhoid fever in children with parenteral ceftriaxone. Scandinavian Journal of Infectious Diseases 1987;19(4):467-8. - PubMed
Gnassingbe 2010 {published data only}
-
- Gnassingbe K, Akakpo-Numado GK, Attipou K, Kanassoua K, Tekou H. Typhoid intestinal perforation in children: renewed interest in the Veillard technique in tropical zones [Les perforations typhiques du grêle chez l'enfant: un regain d'intérêt de la technique de Veillard en milieu tropical]. Médecine Tropicale 2010;70(5-6):524-8. - PubMed
Jiangli 1995 {published data only}
-
- Jiangli Z, Zheng G. Studies on the pharmacodynamics and clinical usage of domestic intravenous preparation of ciprofloxacin lactate. Chinese Journal of Antibiotics 1995;20(2):112-7.
Khichi 2001 {published data only}
-
- Khichi QK, Channer MS. Parenteral chloramphenicol versus chloramphenicol plus dexamethasone in the treatment of typhoid fever. Pakistan Pediatric Journal 2001;25(2):55-8.
Lan 1986 {published data only}
-
- Lan CK, Cheng DL, Lasserre R. Two to three days treatment of typhoid fever with ceftriaxone. Southeast Asian Journal of Tropical Medicine and Public Health 1986;17(1):119-24. - PubMed
Medina 2000 {published data only}
-
- Medina Santillán R, Reyes García G, Herrera Benavente I, Mateos García E. Efficacy of cefixime in the therapy of typhoid fever. Proceedings of the Western Pharmacology Society 2000;43:65-6. - PubMed
Meloni 1988 {published data only}
-
- Meloni T, Marinaro AM, Desole MG, Forteleoni G, Argiolas L. Ceftriaxone treatment of salmonella enteric fever. Pediatric Infectious Disease Journal 1988;7(10):734-5. - PubMed
Morelli 1992 {published data only}
-
- Morelli G, Mazzoli S, Tortoli E, Simonetti MT, Perruna F, Postiglione A. Fluoroquinolones versus chloramphenicol in the therapy of typhoid fever: a clinical and microbiological study. Current Therapeutic Research 1992;52(4):532-42.
Nagaraj 2016 {published data only}
-
- Nagaraj P, Sivanthu S, Manickam K, Kumar S, Kumar S, Sampath S. To study the effectiveness of oral azithromycin as compared to paraenteral ceftriaxone in the treatment of uncomplicated enteric fever. Journal of Pediatric Infectious Diseases 2016;11(4):113-7.
Naveed 2016 {published data only}
-
- Naveed A, Ahmed Z. Treatment of typhoid fever in children: comparison of efficacy of ciprofloxacin with ceftriaxone. European Scientific Journal 2016;12(6):346-55.
Nelson 1967 {published data only}
-
- Nelson JD, Haltalin KC. Broad-spectrum penicillins in enteric infections of children. Annals of the New York Academy of Sciences 1967;145(2):414-22. - PubMed
Park 1985 {published data only}
-
- Park SC, Lee CH, Kim SY, Park CH, Kim TW, Seok SE, et al. Clinical trial of cefotaxime in patients with typhoid fever. Clinical Therapeutics 1985;7(4):448-51. - PubMed
Raoult 1984 {published data only}
-
- Raoult D, Gallais H, Fosse T, Xeridat B, David MF, Casanova P. Treatment of typhoid fever with cefoperazone [Traitement de la fièvre typhoïde par la céfopérazone]. Pathologie Biologie 1984;32(5 Pt 2):573-5. - PubMed
Rolston 1992 {published data only}
-
- Rolston KV, Berkey P, Bodey GP, Anaissie EJ, Khardori NM, Joshi JH, et al. A comparison of imipenem to ceftazidime with or without amikacin as empiric therapy in febrile neutropenic patients. Archives of Internal Medicine 1992;152(2):283-91. - PubMed
Singh 1993 {published data only}
-
- Singh CP, Singh N, Brar GK, Lal G, Kumar H. Efficacy of ciprofloxacin and norfloxacin in multidrug resistant enteric fever in adults. Journal of the Indian Medical Association 1993;91(6):156-7. - PubMed
Sirinavin 2003 {published data only}
-
- Sirinavin S, Thavornnunth J, Sakchainanont B, Bangtrakulnonth A, Chongthawonsatid S, Junumporn S. Norfloxacin and azithromycin for treatment of nontyphoidal salmonella carriers. Clinical Infectious Diseases 2003;37(5):685-91. - PubMed
Soe 1987 {published data only}
-
- Soe GB, Overturf GD. Treatment of typhoid fever and other systemic salmonelloses with cefotaxime, ceftriaxone, cefoperazone and other newer cephalosporins. Reviews of Infectious Diseases 1987;9(4):719-36. - PubMed
Thaver 2009 {published data only}
Ti 1985 {published data only}
Trivedi 2012 {published data only}
-
- Trivedi NA, Shah PC. A meta-analysis comparing the safety and efficacy of azithromycin over the alternate drugs used for treatment of uncomplicated enteric fever. Journal of Postgraduate Medicine 2012;58(2):112-8. - PubMed
Uwaydah 1976 {published data only}
Uwaydah 1984 {published data only}
Vinh 2005 {published data only}
-
- Vinh H, Duong NM, Phuong le T, Truong NT, Bay PV, Wain J, et al. Comparative trial of short-course ofloxacin for uncomplicated typhoid fever in Vietnamese children. Annals of Tropical Paediatrics 2005;25(1):17-22. - PubMed
Yi 1995 {published data only}
-
- Yi L, Huilin Z, Guoyao M, Dungong Q, Peirong H. Clinical study of intravenous enoxacin versus cefotaxime in acute infectious diseases. Chinese Journal of Antibiotics 1995;20(2):123-8.
References to studies awaiting assessment
Amin 2021 {published data only}
-
- Amin MR, Das SK, Kabir A, Islam MR, Ahmed SM, Hasan MJ. Open label randomized controlled comparison of three alternative regimes of ciprofloxacin, azithromycin and cefixime for treatment of uncomplicated typhoid fever in Bangladesh. Mymensingh Medical Journal 2021;30(3):725-37. - PubMed
Hamidullah 2019 {published data only}
-
- Hamidullah, Haq SU, Ahmad I, Ali S. Comparison of the clinical effectiveness of azithromycin versus ceftriaxone in treatment of enteric fever. Medical Forum Monthly 2019;30(1):40-4.
Huai 2000 {published data only}
-
- Huai Y, Zhu Q, Wang X. Ceftriaxone vs norfloxacin in the treatment of resistant typhoid fever in 60 children. Chinese Journal of Pediatrics 2000;38(6):386-8.
Thapaet 2019 {published data only}
-
- Thapaet RK, Raghu PS, Khanal DP. Efficacy of azithromycin and ceftriaxone for the treatment of enteric fever in two tertiary care hospitals of Kathmandu Nepal. Journal of Global Trends in Pharmaceutical Sciences 2019;10(3):6361-7.
References to ongoing studies
NCT04349826 {unpublished data only}
-
- NCT04349826. The azithromycin and cefixime treatment of typhoid in South Asia trial (ACT-South Asia Trial) [Azithromycin and cefixime combination versus azithromycin alone for the out-patient treatment of clinically suspected or confirmed uncomplicated typhoid fever in South Asia; a randomised controlled trial]. clinicaltrials.gov/show/NCT04349826 (first received 16 April 2020).
Additional references
Ahmed 2012
-
- Ahmed D, Hoque A, Mazumder R, Nahar K, Islam N, Gazi SA, et al. Salmonella enterica serovar typhi strain producing extended-spectrum beta-lactamases in Dhaka, Bangladesh. Journal of Medical Microbiology 2012;61(Pt 7):1032-3. - PubMed
Antillón 2017
Baker 2010
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines – 3: rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. - PubMed
Basnyat 2007
Basnyat 2010
-
- Basnyat B. Typhoid fever in the United States and antibiotic choice. Journal of the American Medical Association 2010;303(1):34. - PubMed
Bazan 2011
-
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antibiotics: doripenem, ceftobiprole, ceftaroline, and cefepime. Medical Clinics of North America 2011;95(4):743-60. - PubMed
Bhan 2005
-
- Bhan MK, Bahl R, Bhatnagar S. Typhoid and paratyphoid fever. Lancet 2005;366(9487):749-62. - PubMed
Browne 2020
Buckle 2012
Caygill 1994
-
- Caygill CP, Hill MJ, Braddick M, Sharp JC. Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet 1994;343(8889):83-4. - PubMed
Chatham‐Stephens 2019
CLSI 2021
-
- Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard (M07-A9). 31st edition. Wayne, Pennsylvania: CLSI, 2021.
Crump 2003
-
- Crump JA, Barrett TJ, Nelson JT, Angulo FJ. Reevaluating fluoroquinolone breakpoints for Salmonella enterica serotype typhi and for non-typhi Salmonellae. Clinical Infectious Diseases 2003;37(1):75-81. - PubMed
Crump 2004
Crump 2008
-
- Crump JA, Kretsinger K, Gay K, Hoekstra RM, Vugia DJ, Hurd S, et al. Clinical response and outcome of infection with Salmonella enterica serotype typhi with decreased susceptibility to fluoroquinolones: a United States foodnet multicenter retrospective cohort study. Antimicrobial Agents and Chemotherapy 2008;52(4):1278-84. - PMC - PubMed
Crump 2019
Deeks 2022
-
- Deeks JJ, Higgins JP, Altman DG editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3 (updated February 2022). Available from training.cochrane.org/handbook.
Duy 2020
Effa 2011
Ferreccio 1988
-
- Ferreccio C, Morris JG Jr, Valdivieso C, Prenzel I, Sotomayor V, Drusano GL, et al. Efficacy of ciprofloxacin in the treatment of chronic typhoid carriers. Journal of Infectious Diseases 1988;157(6):1235-9. - PubMed
Garrett 2022
-
- Garrett DO, Longley AT, Aiemjoy K, Yousafzai MT, Hemlock C, Alexander TY, et al. Incidence of typhoid and paratyphoid fever in Bangladesh, Nepal, and Pakistan: results of the Surveillance for Enteric Fever in Asia Project. Lancet Global Health 2022;10(7):e978-88. [DOI: 10.1016/S2214-109X(22)00119-X] - DOI - PMC - PubMed
Gul 2017
Higgins 2003
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. training.cochrane.org/handbook/archive/v5.1/.
Hooda 2019
Iqbal 2020
Kalman 1990
Karkey 2018
-
- Karkey A, Thwaites GE, Baker S. The evolution of antimicrobial resistance in Salmonella typhi. Current Opinion in Gastroenterology 2018;34(1):25-30. - PubMed
Kerr 1992
-
- Kerr JR, Barr JG, Smyth ET, O'Hare J. Techniques for calculation of true costs of antibiotic therapy. European Journal of Clinical Microbiology & Infectious Diseases 1992;11:823-7. - PubMed
Kim 2017
-
- Kim JH, Mogasale V, Im J, Ramani E, Marks F. Updated estimates of typhoid fever burden in sub-Saharan Africa. Lancet Global Health 2017;5(10):e969. - PubMed
Kleine 2017
-
- Kleine CE, Schlabe S, Hischebeth GT, Molitor E, Pfeifer Y, Wasmuth JC, et al. Successful therapy of a multidrug-resistant extended-spectrum beta-lactamase-producing and fluoroquinolone-resistant Salmonella enterica subspecies enterica serovar typhi infection using combination therapy of meropenem and fosfomycin. Clinical Infectious Diseases 2017;65(10):1754-6. - PubMed
Klemm 2018
Levine 1982
-
- Levine MM, Black RE, Lanata C. Precise estimation of the numbers of chronic carriers of Salmonella typhi in Santiago, Chile, an endemic area. Journal of Infectious Diseases 1982;146(6):724-6. - PubMed
Marks 2017
Meiring 2021
Mogasale 2014
-
- Mogasale V, Maskery B, Ochiai RL, Lee JS, Mogasale VV, Ramani E, et al. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Global Health 2014;2(10):e570-80. - PubMed
Munir 2016
-
- Munir T, Lodhi M, Ansari JK, Andleeb S, Ahmed M. Extended spectrum beta lactamase producing cephalosporin resistant Salmonella typhi, reported from Rawalpindi, Pakistan. Journal of the Pakistan Medical Association 2016;66(8):1035-6. - PubMed
Nabarro 2022
Neupane 2021
Parry 1999
Parry 2002
-
- Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. New England Journal of Medicine 2002;347(22):1770-82. - PubMed
Patel 2021
Pfeifer 2009
Qadri 2021
-
- Qadri F, Khanam F, Liu X, Theiss-Nyland K, Biswas PK, Bhuiyan AI, et al. Protection by vaccination of children against typhoid fever with a Vi-tetanus toxoid conjugate vaccine in urban Bangladesh: a cluster-randomised trial. Lancet 2021;398(10301):01124-7. [DOI: 10.1016/S0140-6736] - DOI - PMC - PubMed
Qureshi 2020
Raffatellu 2008
-
- Raffatellu M, Wilson RP, Winter SE, Bäumler AJ. Clinical pathogenesis of typhoid fever. Journal of Infection in Developing Countries 2008;2(4):260-6. - PubMed
RevMan Web 2022 [Computer program]
-
- Review Manager Web (RevMan Web). Version 4.11.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rowe 1997
-
- Rowe B, Ward LR, Threlfall EJ. Multidrug-resistant Salmonella typhi: a worldwide epidemic. Clinical Infectious Diseases 1997;24 Suppl 1:S106-9. - PubMed
Schünemann 2022
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Available from training.cochrane.org/handbook.
Shakya 2021
Sjolund‐Karlsson 2011
Stanaway 2019
Tsolis 1999
-
- Tsolis RM, Kingsley RA, Townsend SM, Ficht TA, Adams LG, Bäumler AJ. Of mice, calves, and men. Comparison of the mouse typhoid model with other Salmonella infections. Advances in Experimental Medicine and Biology 1999;473:261-74. - PubMed
Wain 1998
Wain 2015
Walia 2006
-
- Walia M, Gaind R, Paul P, Mehta R, Aggarwal P, Kalaivani M. Age-related clinical and microbiological characteristics of enteric fever in India. Transactions of the Royal Society of Tropical Medicine and Hygiene 2006;100(10):942-8. - PubMed
WHO 2019
WHO 2021
-
- World Health Organization. Model List of Essential Medicines - 22nd List, 2021. WHO/MHP/HPS/EML/2021.02. who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02.
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

