Retinopathy of prematurity: Metabolic risk factors
- PMID: 36420952
- PMCID: PMC9691009
- DOI: 10.7554/eLife.80550
Retinopathy of prematurity: Metabolic risk factors
Abstract
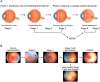
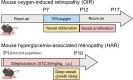
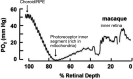
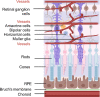
At preterm birth, the retina is incompletely vascularized. Retinopathy of prematurity (ROP) is initiated by the postnatal suppression of physiological retinal vascular development that would normally occur in utero. As the neural retina slowly matures, increasing metabolic demand including in the peripheral avascular retina, leads to signals for compensatory but pathological neovascularization. Currently, only late neovascular ROP is treated. ROP could be prevented by promoting normal vascular growth. Early perinatal metabolic dysregulation is a strong but understudied risk factor for ROP and other long-term sequelae of preterm birth. We will discuss the metabolic and oxygen needs of retina, current treatments, and potential interventions to promote normal vessel growth including control of postnatal hyperglycemia, dyslipidemia and hyperoxia-induced retinal metabolic alterations. Early supplementation of missing nutrients and growth factors and control of supplemental oxygen promotes physiological retinal development. We will discuss the current knowledge gap in retinal metabolism after preterm birth.
Keywords: hyperglycemia; medicine; retinal metabolism; retinopathy of prematurity.
© 2022, Fu et al.
Conflict of interest statement
ZF, AN, AH, LS No competing interests declared
Figures

References
-
- Anand-Apte B, Hollyfield JG. Developmental anatomy of the retinal and choroidal vasculature. Encyclopedia of the Eye. 2010;48:8–9. doi: 10.1016/B978-0-12-374203-2.00169-X. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

