Studying the trafficking of labeled trodusquemine and its application as nerve marker for light-sheet and expansion microscopy
- PMID: 36421008
- PMCID: PMC9827910
- DOI: 10.1096/fj.202201276R
Studying the trafficking of labeled trodusquemine and its application as nerve marker for light-sheet and expansion microscopy
Abstract
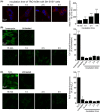
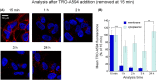
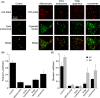
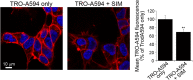
Trodusquemine is an aminosterol with a variety of biological and pharmacological functions, such as acting as an antimicrobial, stimulating body weight loss and interfering with the toxicity of proteins involved in the development of Alzheimer's and Parkinson's diseases. The mechanisms of interaction of aminosterols with cells are, however, still largely uncharacterized. Here, by using fluorescently labeled trodusquemine (TRO-A594 and TRO-ATTO565), we show that trodusquemine binds initially to the plasma membrane of living cells, that the binding affinity is dependent on cholesterol, and that trodusquemine is then internalized and mainly targeted to lysosomes after internalization. We also found that TRO-A594 is able to strongly and selectively bind to myelinated fibers in fixed mouse brain slices, and that it is a marker compatible with tissue clearing and light-sheet fluorescence microscopy or expansion microscopy. In conclusion, this work contributes to further characterize the biology of aminosterols and provides a new tool for nerve labeling suitable for the most advanced microscopy techniques.
Keywords: intracellular trafficking; lipid membrane; nerve fiber staining; neurodegeneration; optical imaging; squalamine.
© 2022 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Figures

References
-
- Rao MN, Shinnar AE, Noecker LA, et al. Aminosterols from the dogfish shark Squalus acanthias. J Nat Prod. 2000;63:631‐635. - PubMed
-
- Wehrli SL, Moore KS, Roder H, Durell S, Zasloff M. Structure of the novel steroidal antibiotic squalamine determined by two‐dimensional NMR spectroscopy. Steroids. 1993;58:370‐378. - PubMed
-
- Sills AK, Williams JI, Tyler BM, et al. Squalamine inhibits angiogenesis and solid tumor growth in vivo and perturbs embryonic vasculature. Cancer Res. 1998;58:2784‐2792. - PubMed
-
- Higgins RD, Sanders RJ, Yan Y, Zasloff M, Williams JI. Squalamine improves retinal neovascularization. Investig Ophthalmol Vis Sci. 2000;41:1507‐1512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

