Biomedical Applications of Microfluidic Devices: A Review
- PMID: 36421141
- PMCID: PMC9688231
- DOI: 10.3390/bios12111023
Biomedical Applications of Microfluidic Devices: A Review
Abstract
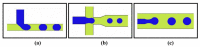
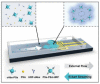
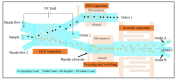
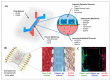
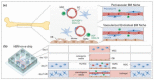
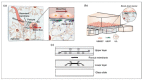
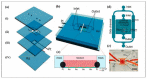
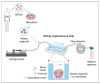
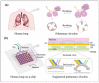
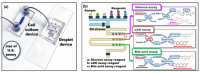
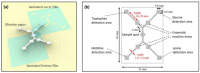
Both passive and active microfluidic chips are used in many biomedical and chemical applications to support fluid mixing, particle manipulations, and signal detection. Passive microfluidic devices are geometry-dependent, and their uses are rather limited. Active microfluidic devices include sensors or detectors that transduce chemical, biological, and physical changes into electrical or optical signals. Also, they are transduction devices that detect biological and chemical changes in biomedical applications, and they are highly versatile microfluidic tools for disease diagnosis and organ modeling. This review provides a comprehensive overview of the significant advances that have been made in the development of microfluidics devices. We will discuss the function of microfluidic devices as micromixers or as sorters of cells and substances (e.g., microfiltration, flow or displacement, and trapping). Microfluidic devices are fabricated using a range of techniques, including molding, etching, three-dimensional printing, and nanofabrication. Their broad utility lies in the detection of diagnostic biomarkers and organ-on-chip approaches that permit disease modeling in cancer, as well as uses in neurological, cardiovascular, hepatic, and pulmonary diseases. Biosensor applications allow for point-of-care testing, using assays based on enzymes, nanozymes, antibodies, or nucleic acids (DNA or RNA). An anticipated development in the field includes the optimization of techniques for the fabrication of microfluidic devices using biocompatible materials. These developments will increase biomedical versatility, reduce diagnostic costs, and accelerate diagnosis time of microfluidics technology.
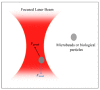
Keywords: acoustophoresis; biomedical applications; biosensors; cancer diagnosis; cell sorting; dielectrophoresis; disease modeling; electrophoresis; lab-on-a-chip; magnetophoresis; micromixers; optical trapping; organ-on-a-chip; particle enrichment; particle separation; point-of-care; pressure fields; thermal fields.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Alijani H., Özbey A., Karimzadehkhouei M., Koşar A. Inertial Micromixing in Curved Serpentine Micromixers with Different Curve Angles. Fluids. 2019;4:204. doi: 10.3390/fluids4040204. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

