Targeting SAM-I Riboswitch Using Antisense Oligonucleotide Technology for Inhibiting the Growth of Staphylococcus aureus and Listeria monocytogenes
- PMID: 36421306
- PMCID: PMC9686682
- DOI: 10.3390/antibiotics11111662
Targeting SAM-I Riboswitch Using Antisense Oligonucleotide Technology for Inhibiting the Growth of Staphylococcus aureus and Listeria monocytogenes
Abstract
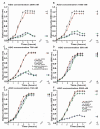
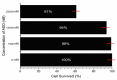
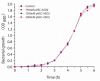
With the discovery of antibiotics, a productive period of antibacterial drug innovation and application in healthcare systems and agriculture resulted in saving millions of lives. Unfortunately, the misusage of antibiotics led to the emergence of many resistant pathogenic strains. Some riboswitches have risen as promising targets for developing antibacterial drugs. Here, we describe the design and applications of the chimeric antisense oligonucleotide (ASO) as a novel antibacterial agent. The pVEC-ASO-1 consists of a cell-penetrating oligopeptide known as pVEC attached to an oligonucleotide part with modifications of the first and the second generations. This combination of modifications enables specific mRNA degradation under multiple turnover conditions via RNase H. The pVEC-ASO targets the S-adenosyl methionine (SAM)-I riboswitch found in the genome of many Gram-positive bacteria. The SAM-I riboswitch controls not only the biosynthesis but also the transport of SAM. We have established an antibiotic dosage of 700 nM (4.5 µg/mL) of pVEC-ASO that inhibits 80% of the growth of Staphylococcus aureus and Listeria monocytogenes. The pVEC-ASO-1 does not show any toxicity in the human cell line at MIC80's concentration. We have proven that the SAM-I riboswitch is a suitable target for antibacterial drug development based on ASO. The approach is rational and easily adapted to other bacterial RNA targets.
Keywords: antibacterial agents; antibacterial drug discovery; antibacterial resistance; cell-penetrating peptides; design of antisense oligonucleotides; drug targets; gram-positive bacteria; riboswitch.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Targeting Bacterial Adenylate Kinase mRNA with a Chimeric Antisense Oligonucleotide for Rational Antibacterial Drug Development.Molecules. 2025 Aug 20;30(16):3425. doi: 10.3390/molecules30163425. Molecules. 2025. PMID: 40871577 Free PMC article.
-
Targeting FMN, TPP, SAM-I, and glmS Riboswitches with Chimeric Antisense Oligonucleotides for Completely Rational Antibacterial Drug Development.Antibiotics (Basel). 2023 Nov 8;12(11):1607. doi: 10.3390/antibiotics12111607. Antibiotics (Basel). 2023. PMID: 37998809 Free PMC article. Review.
-
Targeting TPP Riboswitches Using Chimeric Antisense Oligonucleotide Technology for Antibacterial Drug Development.ACS Appl Bio Mater. 2022 Sep 28. doi: 10.1021/acsabm.2c00628. Online ahead of print. ACS Appl Bio Mater. 2022. PMID: 36170638
-
Engineering Antisense Oligonucleotides as Antibacterial Agents That Target FMN Riboswitches and Inhibit the Growth of Staphylococcus aureus, Listeria monocytogenes, and Escherichia coli.ACS Synth Biol. 2022 May 20;11(5):1845-1855. doi: 10.1021/acssynbio.2c00013. Epub 2022 Apr 20. ACS Synth Biol. 2022. PMID: 35440139
-
[Novel targets for antibiotics discovery: riboswitches].Yao Xue Xue Bao. 2013 Sep;48(9):1361-8. Yao Xue Xue Bao. 2013. PMID: 24358767 Review. Chinese.
Cited by
-
Targeting Bacterial Adenylate Kinase mRNA with a Chimeric Antisense Oligonucleotide for Rational Antibacterial Drug Development.Molecules. 2025 Aug 20;30(16):3425. doi: 10.3390/molecules30163425. Molecules. 2025. PMID: 40871577 Free PMC article.
-
Signals behind Listeria monocytogenes virulence mechanisms.Gut Microbes. 2024 Jan-Dec;16(1):2369564. doi: 10.1080/19490976.2024.2369564. Epub 2024 Jul 9. Gut Microbes. 2024. PMID: 38979800 Free PMC article. Review.
-
The current riboswitch landscape in Clostridioides difficile.Microbiology (Reading). 2024 Oct;170(10):001508. doi: 10.1099/mic.0.001508. Microbiology (Reading). 2024. PMID: 39405103 Free PMC article. Review.
-
Promising strategies employing nucleic acids as antimicrobial drugs.Mol Ther Nucleic Acids. 2024 Jan 18;35(1):102122. doi: 10.1016/j.omtn.2024.102122. eCollection 2024 Mar 12. Mol Ther Nucleic Acids. 2024. PMID: 38333674 Free PMC article. Review.
-
A sensitive and scalable fluorescence anisotropy single stranded RNA targeting approach for monitoring riboswitch conformational states.Nucleic Acids Res. 2024 Apr 12;52(6):3164-3179. doi: 10.1093/nar/gkae118. Nucleic Acids Res. 2024. PMID: 38375901 Free PMC article.
References
-
- Valsamatzi-Panagiotou A., Popova K.B., Penchovsky R. Methods for prevention and constraint of antimicrobial resistance: A review. Environ. Chem. Lett. 2021;19:2005–2012. doi: 10.1007/s10311-021-01206-x. - DOI
-
- Kaji T., Murai M., Itoh H., Yasukawa J., Hamamoto H., Sekimizu K., Inoue M. Total Synthesis and Functional Evaluation of Fourteen Derivatives of Lysocin E: Importance of Cationic, Hydrophobic, and Aromatic Moieties for Antibacterial Activity. Chemistry. 2016;22:16912–16919. doi: 10.1002/chem.201604022. - DOI - PubMed
-
- Pavlova N., Penchovsky R. Genome-wide bioinformatics analysis of FMN, SAM-I, glmS, TPP, lysine, purine, cobalamin, and SAH riboswitches for their applications as allosteric antibacterial drug targets in human pathogenic bacteria. Expert Opin. Ther. Targets. 2019;23:631–643. doi: 10.1080/14728222.2019.1618274. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

