A Robotic Completely Intercorporeal Jejunal Pouch Reconstruction after Gastrectomy
- PMID: 36421331
- PMCID: PMC9689293
- DOI: 10.3390/curroncol29110678
A Robotic Completely Intercorporeal Jejunal Pouch Reconstruction after Gastrectomy
Abstract
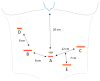
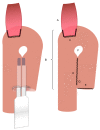
Robotic surgery is increasingly gaining importance. While initial results suggest an advantage of the robotic over the minimally invasive approach in patients with gastric cancer, definitive proof of its superiority has yet to be provided. There are numerous approaches to recreate a gastric reservoir after a total gastrectomy. However, a major disadvantage of most conventional reconstructions are long term effects such as dumping syndrome, afferent loop syndrome and poor nutrition intake with severe impact on the patient quality of life. The jejunal pouch reconstruction is a beneficial reconstruction, which provides a larger reservoir capacity after gastrectomy and prevents anastomotic stenosis and dumping syndrome. The completely intercorporeal approach with a Pfannenstiel incision instead of an unfavorable midline incision can potentially decrease delayed complications such as incision hernias. With the increased deployment of robotic surgery, a complete intercorporeal reconstruction is now possible without major increase in operating time or further technical weak points. We provide for the first time a detailed technical explanation of the completely intercorporeal robotic jejunal pouch reconstruction after gastrectomy.
Keywords: Hunt-Lawrence; Pfannenstiel incision; gastrectomy; gastric cancer; pouch; robot.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Beyer K., Baukloh A.-K., Kamphues C., Seeliger H., Heidecke C.-D., Kreis M.E., Patrzyk M. Laparoscopic versus Open Gastrectomy for Locally Advanced Gastric Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. World J. Surg. Oncol. 2019;17:68. doi: 10.1186/s12957-019-1600-1. - DOI - PMC - PubMed
-
- Park Y.K., Yoon H.M., Kim Y.-W., Park J.Y., Ryu K.W., Lee Y.-J., Jeong O., Yoon K.Y., Lee J.H., Lee S.E., et al. Laparoscopy-Assisted versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: Results From a Randomized Phase II Multicenter Clinical Trial (COACT 1001) Ann. Surg. 2018;267:638–645. doi: 10.1097/SLA.0000000000002168. - DOI - PubMed
-
- Shi Y., Xu X., Zhao Y., Qian F., Tang B., Hao Y., Luo H., Chen J., Yu P. Short-Term Surgical Outcomes of a Randomized Controlled Trial Comparing Laparoscopic versus Open Gastrectomy with D2 Lymph Node Dissection for Advanced Gastric Cancer. Surg. Endosc. 2018;32:2427–2433. doi: 10.1007/s00464-017-5942-x. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

