Influence of Copper-Strontium Co-Doping on Bioactivity, Cytotoxicity and Antibacterial Activity of Mesoporous Bioactive Glass
- PMID: 36421565
- PMCID: PMC9689600
- DOI: 10.3390/gels8110743
Influence of Copper-Strontium Co-Doping on Bioactivity, Cytotoxicity and Antibacterial Activity of Mesoporous Bioactive Glass
Abstract
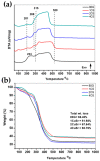
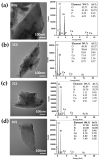
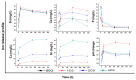
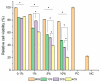
Mesoporous bioactive glass (MBG) is an extensively studied biomaterial used for the healing of bone defects. Its biological applications can be tailored by introducing metallic ions, such as strontium (Sr) and copper (Cu), which can enhance its functionalities, including osteogenetic, angiogenetic and antibacterial functionalities. In this study, Cu and Sr ions were co-doped (ratio 1:1) with x = 0.5, 1 and 2 mol% each in glass with an intended nominal composition of 80SiO2-(15-2x)CaO-5P2O5-xCuO-xSrO and synthesized with an evaporation-induced self-assembly (EISA)-based sol-gel technique. XRD confirmed the amorphous nature of the glass, while compositional analysis using ICP-OES confirmed the presence of dopant ions with the required amounts. A TEM study of the MBG powders showed fringes that corresponded to the formation of a highly ordered mesoporous structure. The Cu-Sr-doped MBG showed a positive effect on apatite formation when immersed in SBF, although the release of Cu and Sr ions was relatively slow for 1 mol% of each co-dopant, which signified a stable network structure in the glass. The impact of the Cu and Sr ions on the osteoblast-like cell line MG-63 was assessed. At the particle concentrations of 1 wt./vol.% or lower, the cell viability was above 50%. An antibacterial test was conducted against Gram-negative E. coli and Gram-positive S. aureus bacteria. With a sequential increase in the co-doped ion content in the glass, the zone of inhibition for bacteria increased. The results suggest that the doping of MBG with Cu and Sr ions at up to 2 mol% can result in tailored sustained release of ions to enhance the applicability of the studied glass as a functional biomaterial for bone regeneration applications.
Keywords: antibacterial activity; bioactivity; co-doping; cytotoxicity; mesoporous glass.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Anand A., Das P., Nandi S.K., Kundu B. Development of antibiotic loaded mesoporous bioactive glass and its drug release kinetics. Ceram. Int. 2020;46:5477–5483. doi: 10.1016/j.ceramint.2019.10.264. - DOI
-
- Zhu Y., Wu C., Ramaswamy Y., Kockrick E., Simon P., Kaskel S., Zreiqat H. Preparation, characterization and in vitro bioactivity of mesoporous bioactive glasses (MBGs) scaffolds for bone tissue engineering. Microporous Mesoporous Mater. 2008;112:494–503. doi: 10.1016/j.micromeso.2007.10.029. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

