In Vitro and Ex Vivo Evaluation of Fluocinolone Acetonide-Acitretin-Coloaded Nanostructured Lipid Carriers for Topical Treatment of Psoriasis
- PMID: 36421568
- PMCID: PMC9689900
- DOI: 10.3390/gels8110746
In Vitro and Ex Vivo Evaluation of Fluocinolone Acetonide-Acitretin-Coloaded Nanostructured Lipid Carriers for Topical Treatment of Psoriasis
Abstract
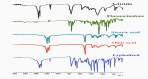
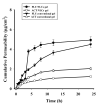
Psoriasis is chronic autoimmune disease that affects 2-5% of the global population. Fluocinolone acetonide (FLU) and acitretin (ACT) are widely used antipsoriatic drugs that belong to BCS classes II and IV, respectively. FLU exhibits side effects, such as skin irritation and a burning sensation. ACT also shows adverse effects, such as gingivitis, teratogenic effects and xerophthalmia. In the present study, topical nanostructured lipid carriers (NLCs) were fabricated to reduce the side effects and enhance the therapeutic efficacy. FLU-ACT-coloaded NLCs were prepared by the modified microemulsion method and optimized by the Box-Behnken model of Design Expert® version 12. The optimization was based on the particle size (PS), zeta potential (ZP) and percentage of encapsulation efficiency (%EE). The physicochemical analyses were performed by TEM, FTIR, XRD and DSC to assess the morphology, chemical interactions between excipients, crystallinity and thermal behavior of the optimized FLU-ACT-coloaded NLCs. The FLU-ACT-coloaded NLCs were successfully loaded into gel and characterized appropriately. The dialysis bag method and Franz diffusion cells were used for the in vitro release and ex vivo permeation studies, respectively. The optimized FLU-ACT-coloaded NLCs had the desired particle size of 288.2 ± 2.3 nm, ZP of -34.2 ± 1.0 mV and %EE values of 81.6 ± 1.1% for ACT and 75 ± 1.3% for FLU. The TEM results confirmed the spherical morphology, while the FTIR results showed the absence of chemical interactions of any type among the ingredients of the FLU-ACT-coloaded NLCs. The XRD and DSC analyses confirmed the amorphous nature and thermal behavior. The in vitro study showed the sustained release of the FLU and ACT from the optimized FLU-ACT-coloaded NLCs and FLU-ACT-coloaded NLC gel compared with the FLU-ACT suspension and conventional gel. The ex vivo study confirmed the minimal permeation of both drugs from the FLU-ACT-coloaded NLC gel.
Keywords: acitretin; bioavailability; entrapment efficiency; fluocinolone acetonide; nanostructured lipid carrier; occlusive effect.
Conflict of interest statement
The authors declare no conflict of interests.
Figures








Similar articles
-
Design, characterization and skin permeating potential of Fluocinolone acetonide loaded nanostructured lipid carriers for topical treatment of psoriasis.Steroids. 2015 Sep;101:56-63. doi: 10.1016/j.steroids.2015.05.012. Epub 2015 Jun 3. Steroids. 2015. PMID: 26049018
-
Transdermal delivery of allopurinol-loaded nanostructured lipid carrier in the treatment of gout.BMC Pharmacol Toxicol. 2022 Nov 28;23(1):86. doi: 10.1186/s40360-022-00625-y. BMC Pharmacol Toxicol. 2022. Retraction in: BMC Pharmacol Toxicol. 2023 Jul 12;24(1):40. doi: 10.1186/s40360-023-00680-z. PMID: 36443818 Free PMC article. Retracted.
-
Fabrication of nanostructured lipid carriers ocugel for enhancing Loratadine used in treatment of COVID-19 related symptoms: statistical optimization, in-vitro, ex-vivo, and in-vivo studies evaluation.Drug Deliv. 2022 Dec;29(1):2868-2882. doi: 10.1080/10717544.2022.2115164. Drug Deliv. 2022. PMID: 36065090 Free PMC article.
-
Mirtazapine Loaded NLCs‑Based Hydrogel for Topical Delivery in Pruritus: Statistical Optimization, In vitro and Skin Irritation Evaluation.Drug Dev Ind Pharm. 2025 Jun;51(6):634-646. doi: 10.1080/03639045.2025.2495846. Epub 2025 Apr 25. Drug Dev Ind Pharm. 2025. PMID: 40262557
-
Formulation and Evaluation of Canagliflozin Hemihydrate-loaded Nanostructured Lipid Carriers Using Box-Behnken Design: Physicochemical Characterization, Ex-vivo Analysis, and In-vivo Pharmacokinetics.Recent Adv Drug Deliv Formul. 2025 Apr 11. doi: 10.2174/0126673878343297250325060051. Online ahead of print. Recent Adv Drug Deliv Formul. 2025. PMID: 40231520
Cited by
-
Pongamia pinnata seed extract-mediated green synthesis of silver nanoparticle loaded nanogel for estimation of their antipsoriatic properties.Bioprocess Biosyst Eng. 2024 Aug;47(8):1409-1431. doi: 10.1007/s00449-024-03058-5. Epub 2024 Jul 12. Bioprocess Biosyst Eng. 2024. PMID: 38995363
-
Novel Sprayable Thermosensitive Benzydamine Hydrogels for Topical Application: Development, Characterization, and In Vitro Biological Activities.AAPS PharmSciTech. 2023 Oct 17;24(8):214. doi: 10.1208/s12249-023-02674-w. AAPS PharmSciTech. 2023. PMID: 37848623
-
Nanostructured Lipid Carriers (NLC)-Based Topical Formulation of Hesperidin for Effective Treatment of Psoriasis.Pharmaceutics. 2025 Apr 7;17(4):478. doi: 10.3390/pharmaceutics17040478. Pharmaceutics. 2025. PMID: 40284473 Free PMC article.
-
Development of Betulin-Loaded Nanostructured Lipid Carriers for the Management of Imiquimod-Induced Psoriasis.AAPS PharmSciTech. 2024 Mar 12;25(3):57. doi: 10.1208/s12249-024-02774-1. AAPS PharmSciTech. 2024. PMID: 38472545
-
QbD-Based Development of Fluocinolone Nanocomposite Transdermal Gel: Optimization, Characterization, and Enhanced Anti-hyperpigmentation Efficacy Assessment.AAPS PharmSciTech. 2025 Apr 2;26(4):100. doi: 10.1208/s12249-025-03094-8. AAPS PharmSciTech. 2025. PMID: 40175791
References
-
- Lomholt G. Prevalence of skin diseases in a population; a census study from the Faroe Islands. Dan. Med. Bull. 1964;11:1–7. - PubMed
LinkOut - more resources
Full Text Sources

