Essential Components of Synthetic Infectious Prion Formation De Novo
- PMID: 36421708
- PMCID: PMC9687555
- DOI: 10.3390/biom12111694
Essential Components of Synthetic Infectious Prion Formation De Novo
Abstract
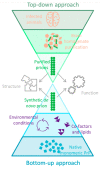
Prion diseases are a class of neurodegenerative diseases that are uniquely infectious. Whilst their general replication mechanism is well understood, the components required for the formation and propagation of highly infectious prions are poorly characterized. The protein-only hypothesis posits that the prion protein (PrP) is the only component of the prion; however, additional co-factors are required for its assembly into infectious prions. These can be provided by brain homogenate, but synthetic lipids and non-coding RNA have also been used in vitro. Here, we review a range of experimental approaches, which generate PrP amyloid assemblies de novo. These synthetic PrP assemblies share some, but not necessarily all, properties of genuine infectious prions. We will discuss the different experimental approaches, how a prion is defined, the non-protein requirements of a prion, and provide an overview of the current state of prion amplification and generation in vitro.
Keywords: PMCA; RT-QuIC; aggregation; infectivity; prions; synthetic prions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
PrP P102L and Nearby Lysine Mutations Promote Spontaneous In Vitro Formation of Transmissible Prions.J Virol. 2017 Oct 13;91(21):e01276-17. doi: 10.1128/JVI.01276-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28835493 Free PMC article.
-
Prion protein amino acid sequence influences formation of authentic synthetic PrPSc.Sci Rep. 2023 Jan 9;13(1):441. doi: 10.1038/s41598-022-26300-0. Sci Rep. 2023. PMID: 36624174 Free PMC article.
-
De novo generation of infectious prions in vitro produces a new disease phenotype.PLoS Pathog. 2009 May;5(5):e1000421. doi: 10.1371/journal.ppat.1000421. Epub 2009 May 15. PLoS Pathog. 2009. PMID: 19436715 Free PMC article.
-
Prion assemblies: structural heterogeneity, mechanisms of formation, and role in species barrier.Cell Tissue Res. 2023 Apr;392(1):149-166. doi: 10.1007/s00441-022-03700-2. Epub 2022 Nov 18. Cell Tissue Res. 2023. PMID: 36399162 Free PMC article. Review.
-
The Prion Concept and Synthetic Prions.Prog Mol Biol Transl Sci. 2017;150:147-156. doi: 10.1016/bs.pmbts.2017.06.002. Epub 2017 Jul 20. Prog Mol Biol Transl Sci. 2017. PMID: 28838659 Review.
Cited by
-
Advanced Situation with Recombinant Toxins: Diversity, Production and Application Purposes.Int J Mol Sci. 2023 Feb 27;24(5):4630. doi: 10.3390/ijms24054630. Int J Mol Sci. 2023. PMID: 36902061 Free PMC article. Review.
-
Clinical value of macrogenome next-generation sequencing on infections.Open Life Sci. 2024 Sep 9;19(1):20220938. doi: 10.1515/biol-2022-0938. eCollection 2024. Open Life Sci. 2024. PMID: 39290502 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

