Extracellular Hemoglobin: Modulation of Cellular Functions and Pathophysiological Effects
- PMID: 36421721
- PMCID: PMC9688122
- DOI: 10.3390/biom12111708
Extracellular Hemoglobin: Modulation of Cellular Functions and Pathophysiological Effects
Abstract
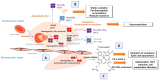
Hemoglobin is essential for maintaining cellular bioenergetic homeostasis through its ability to bind and transport oxygen to the tissues. Besides its ability to transport oxygen, hemoglobin within erythrocytes plays an important role in cellular signaling and modulation of the inflammatory response either directly by binding gas molecules (NO, CO, and CO2) or indirectly by acting as their source. Once hemoglobin reaches the extracellular environment, it acquires several secondary functions affecting surrounding cells and tissues. By modulating the cell functions, this macromolecule becomes involved in the etiology and pathophysiology of various diseases. The up-to-date results disclose the impact of extracellular hemoglobin on (i) redox status, (ii) inflammatory state of cells, (iii) proliferation and chemotaxis, (iv) mitochondrial dynamic, (v) chemoresistance and (vi) differentiation. This review pays special attention to applied biomedical research and the use of non-vertebrate and vertebrate extracellular hemoglobin as a promising candidate for hemoglobin-based oxygen carriers, as well as cell culture medium additive. Although recent experimental settings have some limitations, they provide additional insight into the modulatory activity of extracellular hemoglobin in various cellular microenvironments, such as stem or tumor cells niches.
Keywords: cell culture additives; differentiation; extracellular hemoglobin; hemolysis; oxygen carriers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

