EROS is a selective chaperone regulating the phagocyte NADPH oxidase and purinergic signalling
- PMID: 36421765
- PMCID: PMC9767466
- DOI: 10.7554/eLife.76387
EROS is a selective chaperone regulating the phagocyte NADPH oxidase and purinergic signalling
Abstract
EROS (essential for reactive oxygen species) protein is indispensable for expression of gp91phox, the catalytic core of the phagocyte NADPH oxidase. EROS deficiency in humans is a novel cause of the severe immunodeficiency, chronic granulomatous disease, but its mechanism of action was unknown until now. We elucidate the role of EROS, showing it acts at the earliest stages of gp91phox maturation. It binds the immature 58 kDa gp91phox directly, preventing gp91phox degradation and allowing glycosylation via the oligosaccharyltransferase machinery and the incorporation of the heme prosthetic groups essential for catalysis. EROS also regulates the purine receptors P2X7 and P2X1 through direct interactions, and P2X7 is almost absent in EROS-deficient mouse and human primary cells. Accordingly, lack of murine EROS results in markedly abnormal P2X7 signalling, inflammasome activation, and T cell responses. The loss of both ROS and P2X7 signalling leads to resistance to influenza infection in mice. Our work identifies EROS as a highly selective chaperone for key proteins in innate and adaptive immunity and a rheostat for immunity to infection. It has profound implications for our understanding of immune physiology, ROS dysregulation, and possibly gene therapy.
Keywords: NADPH oxidase;; OST complex; P2X receptor; T cells; chaperone;; immunology; inflammasome; inflammation; mouse.
© 2022, Randzavola, Mortimer et al.
Conflict of interest statement
LR, PM, EG, ED, AS, GR, LY, MP, KS, CT, CB, KH, EN, MN, GW, JW, SS, JS, MM, CS, CK, SM, WR, GD, JG, PL, MC, JC, SC, AS, GS, AB, KS, FM, DT No competing interests declared
Figures

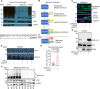
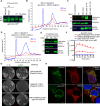
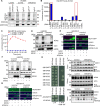
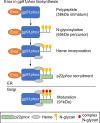
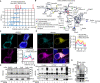
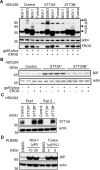




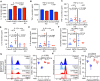
References
-
- Alimchandani M, Lai J-P, Aung PP, Khangura S, Kamal N, Gallin JI, Holland SM, Malech HL, Heller T, Miettinen M, Quezado MM. Gastrointestinal histopathology in chronic granulomatous disease: a study of 87 patients. The American Journal of Surgical Pathology. 2013;37:1365–1372. doi: 10.1097/PAS.0b013e318297427d. - DOI - PMC - PubMed
-
- Arnadottir GA, Norddahl GL, Gudmundsdottir S, Agustsdottir AB, Sigurdsson S, Jensson BO, Bjarnadottir K, Theodors F, Benonisdottir S, Ivarsdottir EV, Oddsson A, Kristjansson RP, Sulem G, Alexandersson KF, Juliusdottir T, Gudmundsson KR, Saemundsdottir J, Jonasdottir A, Jonasdottir A, Sigurdsson A, Manzanillo P, Gudjonsson SA, Thorisson GA, Magnusson OT, Masson G, Orvar KB, Holm H, Bjornsson S, Arngrimsson R, Gudbjartsson DF, Thorsteinsdottir U, Jonsdottir I, Haraldsson A, Sulem P, Stefansson K. A homozygous loss-of-function mutation leading to CYBC1 deficiency causes chronic granulomatous disease. Nature Communications. 2018;9:4447. doi: 10.1038/s41467-018-06964-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

