Neuromorphic-Based Neuroprostheses for Brain Rewiring: State-of-the-Art and Perspectives in Neuroengineering
- PMID: 36421904
- PMCID: PMC9688667
- DOI: 10.3390/brainsci12111578
Neuromorphic-Based Neuroprostheses for Brain Rewiring: State-of-the-Art and Perspectives in Neuroengineering
Abstract
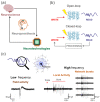
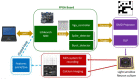
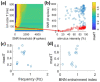
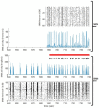
Neuroprostheses are neuroengineering devices that have an interface with the nervous system and supplement or substitute functionality in people with disabilities. In the collective imagination, neuroprostheses are mostly used to restore sensory or motor capabilities, but in recent years, new devices directly acting at the brain level have been proposed. In order to design the next-generation of neuroprosthetic devices for brain repair, we foresee the increasing exploitation of closed-loop systems enabled with neuromorphic elements due to their intrinsic energy efficiency, their capability to perform real-time data processing, and of mimicking neurobiological computation for an improved synergy between the technological and biological counterparts. In this manuscript, after providing definitions of key concepts, we reviewed the first exploitation of a real-time hardware neuromorphic prosthesis to restore the bidirectional communication between two neuronal populations in vitro. Starting from that 'case-study', we provide perspectives on the technological improvements for real-time interfacing and processing of neural signals and their potential usage for novel in vitro and in vivo experimental designs. The development of innovative neuroprosthetics for translational purposes is also presented and discussed. In our understanding, the pursuit of neuromorphic-based closed-loop neuroprostheses may spur the development of novel powerful technologies, such as 'brain-prostheses', capable of rewiring and/or substituting the injured nervous system.
Keywords: brain rewiring; closed-loop; electroceuticals; hybrid neurotechnologies; in vitro; in vivo; neuromorphic; real-time.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


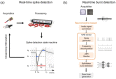
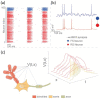
Similar articles
-
A Neuromorphic Prosthesis to Restore Communication in Neuronal Networks.iScience. 2019 Sep 27;19:402-414. doi: 10.1016/j.isci.2019.07.046. Epub 2019 Aug 1. iScience. 2019. PMID: 31421595 Free PMC article.
-
Trends and Challenges in Neuroengineering: Toward "Intelligent" Neuroprostheses through Brain-"Brain Inspired Systems" Communication.Front Neurosci. 2016 Sep 23;10:438. doi: 10.3389/fnins.2016.00438. eCollection 2016. Front Neurosci. 2016. PMID: 27721741 Free PMC article.
-
New Perspectives on Neuroengineering and Neurotechnologies: NSF-DFG Workshop Report.IEEE Trans Biomed Eng. 2016 Jul;63(7):1354-67. doi: 10.1109/TBME.2016.2543662. Epub 2016 Mar 17. IEEE Trans Biomed Eng. 2016. PMID: 27008657
-
Restoration of motor function following spinal cord injury via optimal control of intraspinal microstimulation: toward a next generation closed-loop neural prosthesis.Front Neurosci. 2014 Sep 17;8:296. doi: 10.3389/fnins.2014.00296. eCollection 2014. Front Neurosci. 2014. PMID: 25278830 Free PMC article. Review.
-
Neuromorphic neural interfaces: from neurophysiological inspiration to biohybrid coupling with nervous systems.J Neural Eng. 2017 Aug;14(4):041002. doi: 10.1088/1741-2552/aa67a9. J Neural Eng. 2017. PMID: 28573983 Review.
Cited by
-
Neuromorphic neuromodulation: Towards the next generation of closed-loop neurostimulation.PNAS Nexus. 2024 Oct 30;3(11):pgae488. doi: 10.1093/pnasnexus/pgae488. eCollection 2024 Nov. PNAS Nexus. 2024. PMID: 39554511 Free PMC article. Review.
-
Personalized strategies of neurostimulation: from static biomarkers to dynamic closed-loop assessment of neural function.Front Neurosci. 2024 Mar 7;18:1363128. doi: 10.3389/fnins.2024.1363128. eCollection 2024. Front Neurosci. 2024. PMID: 38516316 Free PMC article. Review.
-
Neuromorphic algorithms for brain implants: a review.Front Neurosci. 2025 Apr 11;19:1570104. doi: 10.3389/fnins.2025.1570104. eCollection 2025. Front Neurosci. 2025. PMID: 40292025 Free PMC article. Review.
-
NRV: An open framework for in silico evaluation of peripheral nerve electrical stimulation strategies.PLoS Comput Biol. 2024 Jul 12;20(7):e1011826. doi: 10.1371/journal.pcbi.1011826. eCollection 2024 Jul. PLoS Comput Biol. 2024. PMID: 38995970 Free PMC article.
-
Living-Neuron-Based Autogenerator.Sensors (Basel). 2023 Aug 8;23(16):7016. doi: 10.3390/s23167016. Sensors (Basel). 2023. PMID: 37631552 Free PMC article.
References
-
- WHO. The Top 10 Causes of Death. World Health Oraganization. Dec 9, 2020.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

