Personalised Treatment in Aortic Stenosis: A Patient-Tailored Transcatheter Aortic Valve Implantation Approach
- PMID: 36421942
- PMCID: PMC9694505
- DOI: 10.3390/jcdd9110407
Personalised Treatment in Aortic Stenosis: A Patient-Tailored Transcatheter Aortic Valve Implantation Approach
Abstract
Transcatheter aortic valve replacement (TAVI) has become a game changer in the management of severe aortic stenosis shifting the concept from inoperable or high-risk patients to intermediate or low surgical-risk individuals. Among devices available nowadays, there is no clear evidence that one device is better than the other or that one device is suitable for all patients. The selection of the optimal TAVI valve for every patient represents a challenging process for clinicians, given a large number of currently available devices. Consequently, understanding the advantages and disadvantages of each valve and personalising the valve selection based on patient-specific clinical and anatomical characteristics is paramount. This review article aims to both analyse the available devices in the presence of specific clinical and anatomic features and offer guidance to select the most suitable valve for a given patient.
Keywords: balloon expandable valves (BEV); self-expandable valves (SEV); surgical aortic valve replacement (SAVR); transcatheter aortic valve implantation (TAVI); transcatheter aortic valve replacement (TAVR).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
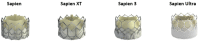



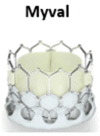
References
-
- Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., Gentile F., Jneid H., Krieger E.V., Mack M., McLeod C., et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e35–e71. doi: 10.1161/CIR.0000000000000932. - DOI - PubMed
-
- Pellegrini C., Rheude T., Michel J., Alvarez-Covarrubias H.A., Wünsch S., Mayr N.P., Xhepa E., Kastrati A., Schunkert H., Joner M., et al. Comparison of Latest Generation Supra-Annular and Intra-Annular Self-Expanding Transcatheter Heart Valves. J. Thorac. Dis. 2020;12:6769–6779. doi: 10.21037/jtd-20-1700. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

