SARS-CoV-2-Induced Amyloidgenesis: Not One, but Three Hypotheses for Cerebral COVID-19 Outcomes
- PMID: 36422238
- PMCID: PMC9692683
- DOI: 10.3390/metabo12111099
SARS-CoV-2-Induced Amyloidgenesis: Not One, but Three Hypotheses for Cerebral COVID-19 Outcomes
Abstract
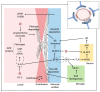
The main neuropathological feature of Alzheimer's disease (AD) is extracellular amyloid deposition in senile plaques, resulting from an imbalance between the production and clearance of amyloid beta peptides. Amyloid deposition is also found around cerebral blood vessels, termed cerebral amyloid angiopathy (CAA), in 90% of AD cases. Although the relationship between these two amyloid disorders is obvious, this does not make CAA a characteristic of AD, as 40% of the non-demented population presents this derangement. AD is predominantly sporadic; therefore, many factors contribute to its genesis. Herein, the starting point for discussion is the COVID-19 pandemic that we are experiencing and how SARS-CoV-2 may be able to, both directly and indirectly, contribute to CAA, with consequences for the outcome and extent of the disease. We highlight the role of astrocytes and endothelial cells in the process of amyloidgenesis, as well as the role of other amyloidgenic proteins, such as fibrinogen and serum amyloid A protein, in addition to the neuronal amyloid precursor protein. We discuss three independent hypotheses that complement each other to explain the cerebrovascular amyloidgenesis that may underlie long-term COVID-19 and new cases of dementia.
Keywords: Aβ; COVID-19; SARS-CoV-2; amyloid; astrocyte; fibrin; serum amyloid A.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Najjar S., Najjar A., Chong D.J., Pramanik B.K., Kirsch C., Kuzniecky R.I., Pacia S.V., Azhar S. Central Nervous System Complications Associated with SARS-CoV-2 Infection: Integrative Concepts of Pathophysiology and Case Reports. J. Neuroinflamm. 2020;17:231. doi: 10.1186/s12974-020-01896-0. - DOI - PMC - PubMed
-
- Premraj L., Kannapadi N.V., Briggs J., Seal S.M., Battaglini D., Fanning J., Suen J., Robba C., Fraser J., Cho S.-M. Mid and Long-Term Neurological and Neuropsychiatric Manifestations of Post-COVID-19 Syndrome: A Meta-Analysis. J. Neurol. Sci. 2022;434:120162. doi: 10.1016/j.jns.2022.120162. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

