Expression Analysis of Molecular Chaperones Hsp70 and Hsp90 on Development and Metabolism of Different Organs and Testis in Cattle (Cattle-yak and Yak)
- PMID: 36422254
- PMCID: PMC9694778
- DOI: 10.3390/metabo12111114
Expression Analysis of Molecular Chaperones Hsp70 and Hsp90 on Development and Metabolism of Different Organs and Testis in Cattle (Cattle-yak and Yak)
Abstract
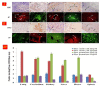
Hsp70 and Hsp90 play an important role in testis development and spermatogenesis regulation, but the exact connection between Hsp70 and Hsp90 and metabolic stress in cattle is unclear. Here, we focused on the male cattle−yak and yak, investigated the expression and localization of Hsp70 and Hsp90 in their tissues, and explored the influence of these factors on development and metabolism. In our study, a total of 54 cattle (24 cattle−yaks and 30 yaks; aged 1 day to 10 years) were examined. The Hsp90 mRNA of the cattle−yak was first cloned and compared with that of the yak, and variation in the amino acid sequence was found, which led to differences in protein spatial structure. Using real-time quantitative PCR (RT-qPCR) and Western blot (WB) techniques, we investigated whether the expression of Hsp70 and Hsp90 mRNA and protein are different in the cattle−yak and yak. We found a disparity in Hsp70 and Hsp90 mRNA and protein expression in different non-reproductive organs and in testicular tissues at different stages of development, while high expression was observed in the testes of both juveniles and adults. Moreover, it was intriguing to observe that Hsp70 expression was significantly high in the yak, whereas Hsp90 was high in the cattle−yak (p < 0.01). We also examined the location of Hsp70 and Hsp90 in the testis by immunohistochemical (IHC) and immunofluorescence (IF) techniques, and the results showed that Hsp70 and Hsp90 were positive in the epithelial cells, spermatogenic cells, and mesenchymal cells. In summary, our study proved that Hsp70 and Hsp90 expressions were different in different tissues (kidney, heart, cerebellum, liver, lung, spleen, and testis), and Hsp90 expression was high in the testis of the cattle−yak, suggesting that dysplasia of the cattle−yak may correlate with an over-metabolism of Hsp90.
Keywords: Hsp70 and Hsp90; cattle; different organs; expression; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Wiener G., Jianlin H., Ruijun L. The Yak. FAO Regional Office for Asia and the Pacific; Bangkok, Thailand: 2003.
-
- Liu P.G., Yu S.J., Cui Y., He J.F., Zhang Q., Sun J., Huang Y.F., Yang X.Q., Cao M.X., Liao B., et al. Regulation by Hsp27/P53 in testis development and sperm apoptosis of male cattle (cattle-yak and yak) J. Cell. Physiol. 2018;234:650–660. - PubMed
-
- Shen H., Fan X.R., Zhang Z., Xi H.M., Ji R., Liu Y.H., Yue M.S., Li Q.H., He J.P. Effects of elevated ambient temperature and local testicular heating on the expressions of heat shock protein 70 and androgen receptor in boar testes. Acta Histochem. 2019;121:297–302. doi: 10.1016/j.acthis.2019.01.009. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

