5-Aza-2'-Deoxycytidine Regulates White Adipocyte Browning by Modulating miRNA-133a/Prdm16
- PMID: 36422269
- PMCID: PMC9695087
- DOI: 10.3390/metabo12111131
5-Aza-2'-Deoxycytidine Regulates White Adipocyte Browning by Modulating miRNA-133a/Prdm16
Abstract
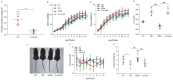
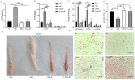
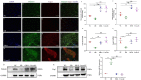
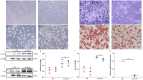
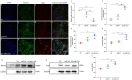
The conversion of white adipocytes into brown adipocytes improves their thermogenesis and promotes energy consumption. Epigenetic modifications affect related genes and interfere with energy metabolism, and these are the basis of new ideas for obesity treatment. Neonatal mice show high levels of DNA hypermethylation in white adipose tissue early in life and low levels in brown adipose tissue. Thus, we considered that the regulation of DNA methylation may play a role in the conversion of white adipose to brown. We observed growth indicators, lipid droplets of adipocytes, brown fat specific protein, and miRNA-133a after treatment with 5-Aza-2'-deoxycytidine. The expression of Prdm16 and Ucp-1 in adipocytes was detected after inhibiting miRNA-133a. The results showed a decrease in total lipid droplet formation and an increased expression of the brown fat specific proteins Prdm16 and Ucp-1. This study indicated that 5-Aza-2'-deoxycytidine promotes white adipocyte browning following DNA demethylation, possibly via the modulation of miR-133a and Prdm16.
Keywords: 5-Aza-dC; Prdm16; Ucp-1; brown adipocytes; white adipocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Reversine promotes browning of white adipocytes by suppressing miR-133a.J Cell Physiol. 2019 Apr;234(4):3800-3813. doi: 10.1002/jcp.27148. Epub 2018 Aug 21. J Cell Physiol. 2019. PMID: 30132867
-
miR-133a regulates adipocyte browning in vivo.PLoS Genet. 2013;9(7):e1003626. doi: 10.1371/journal.pgen.1003626. Epub 2013 Jul 11. PLoS Genet. 2013. PMID: 23874225 Free PMC article.
-
Two key temporally distinguishable molecular and cellular components of white adipose tissue browning during cold acclimation.J Physiol. 2015 Aug 1;593(15):3267-80. doi: 10.1113/JP270805. Epub 2015 Jul 14. J Physiol. 2015. PMID: 26096127 Free PMC article.
-
Role of PRDM16 in the activation of brown fat programming. Relevance to the development of obesity.Histol Histopathol. 2013 Nov;28(11):1411-25. doi: 10.14670/HH-28.1411. Epub 2013 Jun 17. Histol Histopathol. 2013. PMID: 23771475 Review.
-
PRDM16 Regulating Adipocyte Transformation and Thermogenesis: A Promising Therapeutic Target for Obesity and Diabetes.Front Pharmacol. 2022 Apr 8;13:870250. doi: 10.3389/fphar.2022.870250. eCollection 2022. Front Pharmacol. 2022. PMID: 35462933 Free PMC article. Review.
Cited by
-
Increased FGF-21 Improves Ectopic Lipid Deposition in the Liver and Skeletal Muscle.Nutrients. 2024 Apr 23;16(9):1254. doi: 10.3390/nu16091254. Nutrients. 2024. PMID: 38732501 Free PMC article.
-
DNA methylation and demethylation in adipocyte biology: roles of DNMT and TET proteins in metabolic disorders.Front Endocrinol (Lausanne). 2025 Jun 20;16:1591152. doi: 10.3389/fendo.2025.1591152. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40620794 Free PMC article. Review.
-
Pulling the trigger: Noncoding RNAs in white adipose tissue browning.Rev Endocr Metab Disord. 2024 Apr;25(2):399-420. doi: 10.1007/s11154-023-09866-6. Epub 2023 Dec 29. Rev Endocr Metab Disord. 2024. PMID: 38157150 Review.
References
-
- Petrovic N., Walden T.B., Shabalina I.G., Timmons J.A., Cannon B., Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP-1containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 2010;285:7153–7164. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

