Natural Products Targeting Hsp90 for a Concurrent Strategy in Glioblastoma and Neurodegeneration
- PMID: 36422293
- PMCID: PMC9697676
- DOI: 10.3390/metabo12111153
Natural Products Targeting Hsp90 for a Concurrent Strategy in Glioblastoma and Neurodegeneration
Abstract
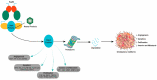
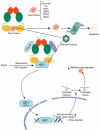
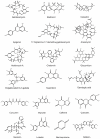
Glioblastoma multiforme (GBM) is one of the most common aggressive, resistant, and invasive primary brain tumors that share neurodegenerative actions, resembling many neurodegenerative diseases. Although multiple conventional approaches, including chemoradiation, are more frequent in GBM therapy, these approaches are ineffective in extending the mean survival rate and are associated with various side effects, including neurodegeneration. This review proposes an alternative strategy for managing GBM and neurodegeneration by targeting heat shock protein 90 (Hsp90). Hsp90 is a well-known molecular chaperone that plays essential roles in maintaining and stabilizing protein folding to degradation in protein homeostasis and modulates signaling in cancer and neurodegeneration by regulating many client protein substrates. The therapeutic benefits of Hsp90 inhibition are well-known for several malignancies, and recent evidence highlights that Hsp90 inhibitors potentially inhibit the aggressiveness of GBM, increasing the sensitivity of conventional treatment and providing neuroprotection in various neurodegenerative diseases. Herein, the overview of Hsp90 modulation in GBM and neurodegeneration progress has been discussed with a summary of recent outcomes on Hsp90 inhibition in various GBM models and neurodegeneration. Particular emphasis is also given to natural Hsp90 inhibitors that have been evidenced to show dual protection in both GBM and neurodegeneration.
Keywords: Hsp90; chemosensitivity; glioblastoma; neurodegenerations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- McCarthy N. Rooting out resistance. Nat. Rev. Cancer. 2006;6:904. doi: 10.1038/nrc2031. - DOI
-
- Minniti G., Muni R., Lanzetta G., Marchetti P., Enrici R.M. Chemotherapy for glioblastoma: Current treatment and future perspectives for cytotoxic and targeted agents. Anticancer Res. 2009;29:5171–5184. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

