The Effect of the Gallbladder Environment during Chronic Infection on Salmonella Persister Cell Formation
- PMID: 36422346
- PMCID: PMC9698170
- DOI: 10.3390/microorganisms10112276
The Effect of the Gallbladder Environment during Chronic Infection on Salmonella Persister Cell Formation
Abstract
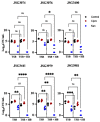
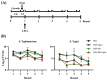
Typhoid fever is caused by Salmonella enterica serovar Typhi (S. Typhi). Around 3-5% of individuals infected become chronic carriers, with the gallbladder (GB) as the predominant site of persistence. Gallstones (GS) aid in the development and maintenance of GB carriage, serving as a substrate to which Salmonellae attach and form a biofilm. This biofilm matrix protects bacteria from the host immune system and environmental stress. This shielded environment is an ideal place for the development of persister cells, a transient phenotype of a subset of cells within a population that allows survival after antibiotic treatment. Persisters can also arise in response to harsh environments such as the GB. Here we investigate if GB conditions affect the number of persisters in a Salmonella population. To simulate the chronic GB environment, we cultured biofilms in cholesterol-coated 96-well plates in the presence of ox or human bile. We then treated planktonic or biofilm Salmonella cultures with high concentrations of different antibiotics. This study suggests that biofilms provide a niche for persister cells, but GB conditions either play no role or have a negative influence on persister formation, especially after kanamycin treatment. The antibiotic target was important, as antimicrobials directed against DNA replication or the cell wall had no effect on persister cell formation. Interestingly, repeated treatment with ciprofloxacin increased the percentage of S. Typhimurium persisters in a biofilm, but this increase was abolished by GB conditions. On the other hand, repeated ciprofloxacin treatment of S. Typhi biofilms in GB conditions slightly increased the fraction of persisters. Thus, while the harsh conditions in the GB would be thought to give rise to increased persisters, therefore contributing to the development of chronic carriage, these data suggest persister cell formation is dampened in this environment.
Keywords: Salmonella; bile; biofilm; persisters; typhoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Human Bile-Mediated Regulation of Salmonella Curli Fimbriae.J Bacteriol. 2019 Aug 22;201(18):e00055-19. doi: 10.1128/JB.00055-19. Print 2019 Sep 15. J Bacteriol. 2019. PMID: 30936374 Free PMC article.
-
A temporary cholesterol-rich diet and bacterial extracellular matrix factors favor Salmonella spp. biofilm formation in the cecum.mBio. 2025 Jan 8;16(1):e0324224. doi: 10.1128/mbio.03242-24. Epub 2024 Dec 5. mBio. 2025. PMID: 39636114 Free PMC article.
-
Establishment of Chronic Typhoid Infection in a Mouse Carriage Model Involves a Type 2 Immune Shift and T and B Cell Recruitment to the Gallbladder.mBio. 2019 Oct 1;10(5):e02262-19. doi: 10.1128/mBio.02262-19. mBio. 2019. PMID: 31575775 Free PMC article.
-
Multidrug tolerance of biofilms and persister cells.Curr Top Microbiol Immunol. 2008;322:107-31. doi: 10.1007/978-3-540-75418-3_6. Curr Top Microbiol Immunol. 2008. PMID: 18453274 Review.
-
Biofilm Producing Salmonella Typhi: Chronic Colonization and Development of Gallbladder Cancer.Int J Mol Sci. 2017 Aug 31;18(9):1887. doi: 10.3390/ijms18091887. Int J Mol Sci. 2017. PMID: 28858232 Free PMC article. Review.
Cited by
-
Targeting lon protease to inhibit persister cell formation in Salmonella Typhimurium: a drug repositioning approach.Front Cell Infect Microbiol. 2024 Sep 5;14:1427312. doi: 10.3389/fcimb.2024.1427312. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39301287 Free PMC article.
-
The footprint of gut microbiota in gallbladder cancer: a mechanistic review.Front Cell Infect Microbiol. 2024 May 7;14:1374238. doi: 10.3389/fcimb.2024.1374238. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38774627 Free PMC article. Review.
References
-
- Chambers H., McPhee S., Papadakis M., Tierney L. Current Medical Diagnosis and Treatment. McGraw-Hill Medical; New York, NY, USA: 2008. [(accessed on 25 August 2021)]. Available online: https://scholar.google.com/scholar_lookup?title=Current%20Medical%20Diag....
-
- Ristori C., Rodríguez H., Vicent P., Ferreccio C., García J., Lobos H., D’Ottone K. Persistence of the Salmonella typhi-paratyphi carrier state after gallbladder removal. Bull. Pan Am. Health Organ. 1982;16:361–366. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

