Development of 3D Printed Enzymatic Microreactors for Lipase-Catalyzed Reactions in Deep Eutectic Solvent-Based Media
- PMID: 36422383
- PMCID: PMC9693471
- DOI: 10.3390/mi13111954
Development of 3D Printed Enzymatic Microreactors for Lipase-Catalyzed Reactions in Deep Eutectic Solvent-Based Media
Abstract
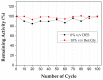
In this study, 3D printing technology was exploited for the development of immobilized enzyme microreactors that could be used for biocatalytic processes in Deep Eutectic Solvent (DES)-based media. 3D-printed polylactic acid (PLA) microwell plates or tubular microfluidic reactors were modified with polyethylenimine (PEI) and lipase from Candida antarctica (CALB) was covalently immobilized in the interior of each structure. DESs were found to have a negligible effect on the activity and stability of CALB, and the system proved highly stable and reusable in the presence of DESs for the hydrolysis of p-nitrophenyl butyrate (p-NPB). A kinetic study under flow conditions revealed an enhancement of substrate accessibility in the presence of Betaine: Glycerol (Bet:Gly) DES, while the system was not severely affected by diffusion limitations. Incubation of microreactors in 100% Bet:Gly preserved the enzyme activity by 53% for 30 days of storage at 60 °C, while the buffer-stored sample had already been deactivated. The microfluidic enzyme reactor was efficiently used for the trans-esterification of ethyl ferulate (EF) with glycerol towards the production of glyceryl ferulate (GF), known for its antioxidant potential. The biocatalytic process under continuous flow conditions exhibited 23 times higher productivity than the batch reaction system. This study featured an effective and robust biocatalytic system with immobilized lipase that can be used both in hydrolytic and synthetic applications, while further optimization is expected to upgrade the microreactor system performance.
Keywords: 3D printing; deep eutectic solvents; enzyme microreactor; immobilization; lipase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Basso A., Serban S. Industrial Applications of Immobilized Enzymes—A Review. Mol. Catal. 2019;479:110607. doi: 10.1016/j.mcat.2019.110607. - DOI
LinkOut - more resources
Full Text Sources

