Microglial iron trafficking: new player in brain injury
- PMID: 36422479
- PMCID: PMC10395700
- DOI: 10.55730/1300-0144.5481
Microglial iron trafficking: new player in brain injury
Abstract
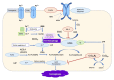
Neonatal brain injury is a significant reason of neurodevelopmental abnormalities and long-term neurological impairments. Hypoxic-ischemic encephalopathy and preterm brain injury, including intraventricular hemorrhage are the most common grounds of brain injury for full-term and preterm neonates. The prevalence of hypoxic ischemic encephalopathy varies globally, ranging from 1 to 3.5/1000 live births in high-resource countries and 26/1000 in low-resource countries. Preterm birth's global incidence is 15 million, a significant reason for infant mortality and morbidity, permanent neurologic problems, and the associated social and economic burden. The widespread neurodevelopmental effects of neonatal brain injury could have an unfavorable impact on a variety of aspects of cognitive, linguistic, behavioral, sensory, and motor functions. Brain injury occurs via various mechanisms, including energy deprivation, excitatory amino acids, mitochondrial dysfunction, reactive oxygen species, and inflammation giving rise to different forms of cell death. The contribution of microglial activity in neonatal brain injury has widely been underlined by focusing on cell death mechanisms since the neuronal death pathways during their development are distinct from those in the adult brain. Iron accumulation and lipid peroxidation cause a relatively novel type of regulated cell death called ferroptosis. Neonates generally have biochemical iron inequalities, and their antioxidant potential is highly restricted, implying that ferroptosis may be significant in pathologic conditions. Moreover, inhaled nitric oxide therapy in infants may lead to microglial inflammation via ferroptosis and neuronal injury in the developing brain. This review article aims to summarize the studies that investigated the association between neonatal brain injury and iron metabolism, with a particular emphasis on the microglial activity and its application to the inhibition of neonatal brain injury.
Keywords: Neonatal brain development; brain injury in neonates; ferroptosis; ipid peroxidation; iron metabolism; microglia.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Gale C, Statnikov Y, Jawad S, Uthaya SN, Modi N, et al. Neonatal brain injuries in England: population-based incidence derived from routinely recorded clinical data held in the National Neonatal Research Database. Archives of Disease in Childhood: Fetal & Neonatal Edition. 2018;103(4):F301–F306. doi: 10.1136/archdischild-2017-313707. - DOI - PMC - PubMed
-
- Robertson L, Knight H, Prosser Snelling E, Petch E, Knight M, et al. Each baby counts: National quality improvement programme to reduce intrapartum-related deaths and brain injuries in term babies. Seminars in Fetal and Neonatal Medicine. 2017;22(3):193–198. doi: 10.1016/j.siny.2017.02.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

