Long-Term Efficacy and Safety of Evinacumab in Patients with Homozygous Familial Hypercholesterolemia: Real-World Clinical Experience
- PMID: 36422519
- PMCID: PMC9698659
- DOI: 10.3390/ph15111389
Long-Term Efficacy and Safety of Evinacumab in Patients with Homozygous Familial Hypercholesterolemia: Real-World Clinical Experience
Abstract
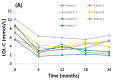
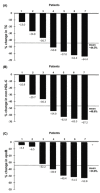
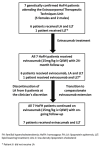
Homozygous familial hypercholesterolemia (HoFH) is a rare, genetic condition characterized by markedly elevated plasma low-density lipoprotein cholesterol (LDL-C) concentrations from birth and increased risk of premature atherosclerotic cardiovascular disease. Evinacumab is an inhibitor of angiopoietin-like 3 protein that offers a new approach for correcting high LDL-C in HoFH. Evinacumab was administered intravenously (15 mg/kg Q4W) for 24 months in 7 patients with genetically confirmed HoFH, receiving background lipoprotein apheresis (LA) and/or lipid-lowering treatment (LLT). Assessment of efficacy and safety were carried out before and after 24 months of evinacumab treatment. The LDL-C lowering effect of evinacumab without LA were also investigated in the 7 HoFH patients after a subsequent compassionate extension period. Twenty-four months of treatment with evinacumab against background LA and LLT resulted in a significant reduction in LDL-C (−46.8%; p < 0.001). LDL-C reduction with evinacumab was maintained during the compassionate extensions period in the absence of treatment with LA (−43.4%; mean follow-up of 208 ± 90 days). Evinacumab was well-tolerated, with no major adverse event reported or significant changes in liver and muscle enzyme concentrations. Our findings suggest that evinacumab is a safe and effective treatment for patients with HoFH receiving best standard of care in a routine setting.
Keywords: angiopoietin-like 3 protein inhibitors; atherosclerotic cardiovascular disease; familial hypercholesterolemia; lipoprotein apheresis; low-density lipoprotein.
Conflict of interest statement
CS has received honoraria for consultancy and speaking engagements from Aegerion, Fresenius Medical Care and Kaneka NV. GFW has received honoraria for advisory boards and research grants from Amgen, Arrowhead, Esperion, AstraZeneca, Kowa, Novartis, Pfizer, Sanofi and Regeneron. DC, SDG and CM declare no conflict of interest.
Figures





References
-
- Cuchel M., Bruckert E., Ginsberg H.N., Raal F.J., Santos R.D., Hegele R.A., Kuivenhoven J.A., Nordestgaard B.G., Descamps O.S., Steinhagen-Thiessen E. Homozygous familial hypercholesterolaemia: New insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur. Heart J. 2014;35:2146–2157. doi: 10.1093/eurheartj/ehu274. - DOI - PMC - PubMed
-
- Moorjani S., Roy M., Torres A., Bétard C., Gagné C., Lambert M., Brun D., Davignon J., Lupien P. Mutations of low-density-lipoprotein-receptor gene, variation in plasma cholesterol, and expression of coronary heart disease in homozygous familial hypercholesterolaemia. Lancet. 1993;341:1303–1306. doi: 10.1016/0140-6736(93)90815-X. - DOI - PubMed
-
- Tromp T.R., Hartgers M.L., Hovingh G.K., Vallejo-Vaz A.J., Ray K.K., Soran H., Freiberger T., Bertolini S., Harada-Shiba M., Blom D.J., et al. Homozygous familial hypercholesterolaemia International Clinical Collaborators. Worldwide experience of homozygous familial hypercholesterolaemia: Retrospective cohort study. Lancet. 2022;399:719–728. doi: 10.1016/S0140-6736(21)02001-8. - DOI - PMC - PubMed
-
- Raal F.J., Pilcher G.J., Panz V.R., van Deventer H.E., Brice B.C., Blom D.J., Marais A.D. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation. 2011;124:2202–2207. doi: 10.1161/CIRCULATIONAHA.111.042523. - DOI - PubMed
LinkOut - more resources
Full Text Sources

