Nitro-Containing Self-Immolative Systems for Biological Applications
- PMID: 36422534
- PMCID: PMC9695724
- DOI: 10.3390/ph15111404
Nitro-Containing Self-Immolative Systems for Biological Applications
Abstract
Since its introduction in 1981, the chemistry of self-immolative systems has received increasing attention in different application areas, such as analytical chemistry, medicinal chemistry, and materials science. This strategy is based on a stimulation that triggers a cascade of disassembling reactions leading to the release of smaller molecules. The particular reactivity of the nitro group, due to its powerful electron-withdrawing nature, has been exploited in the field of self-immolative chemistry. In this context, the present review describes the major role of the nitro group in self-immolative processes depending on its position.
Keywords: nitro; probe; prodrug; self-immolation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
























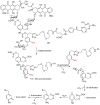

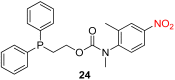
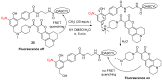


References
Publication types
LinkOut - more resources
Full Text Sources

