Osmotic Pump Drug Delivery Systems-A Comprehensive Review
- PMID: 36422560
- PMCID: PMC9697821
- DOI: 10.3390/ph15111430
Osmotic Pump Drug Delivery Systems-A Comprehensive Review
Abstract
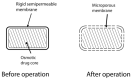
In the last couple of years, novel drug delivery systems (NDDS) have attracted much attention in the food and pharmaceutical industries. NDDS is a broad term that encompasses many dosage forms, one of which is osmotic pumps. Osmotic pumps are considered to be the most reliable source of controlled drug delivery, both in humans and in animals. These pumps are osmotically controlled and release active agents through osmotic pressure. To a large extent, drug release from such a system is independent of gastric fluids. Based on such unique properties and advantages, osmotic pumps have made their mark on the pharmaceutical industry. This review summarizes the available osmotic devices for implantation and osmotic tablets for oral administration.
Keywords: controlled release system; novel drug delivery system; osmosis; osmotic pressure; osmotic pump.
Conflict of interest statement
The author (YA) declares no conflict of interest.
Figures


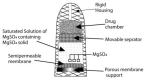
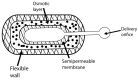

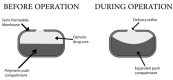
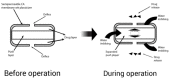

References
-
- Kaparissides, Costas, Alexandridou S., Kotti K., Chaitidou S. Recent advances in novel drug delivery systems. J. Nanotechnol. Online. 2006;2:1–11.
-
- Patel J., Parikh S., Patel S. Comprehensive review on osmotic drug delivery system. World J. Pharm. Res. 2021;10:29.
-
- Bruschi M.L. Strategies to Modify the Drug Release from Pharmaceutical Systems. Woodhead Publishing; Sawston, UK: 2015.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

