The Role of Dendritic Cells in the Host Response to Marek's Disease Virus (MDV) as Shown by Transcriptomic Analysis of Susceptible and Resistant Birds
- PMID: 36422592
- PMCID: PMC9698451
- DOI: 10.3390/pathogens11111340
The Role of Dendritic Cells in the Host Response to Marek's Disease Virus (MDV) as Shown by Transcriptomic Analysis of Susceptible and Resistant Birds
Abstract
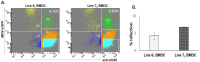
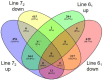
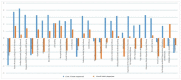
Despite the successful control of highly contagious tumorigenic Marek's disease (MD) by vaccination, a continuous increase in MD virus (MDV) virulence over recent decades has put emphasis on the development of more MD-resistant chickens. The cell types and genes involved in resistance therefore need to be recognized. The virus is primarily lymphotropic, but research should also focus on innate immunity, as innate immune cells are among the first to encounter MDV. Our previous study on MDV-macrophage interaction revealed significant differences between MHC-congenic lines 61 (MD-resistant) and 72 (MD-susceptible). To investigate the role of dendritic cells (DCs) in MD resistance, bone-marrow-derived DCs from these lines were infected with MDV in vitro. They were then characterized by cell sorting, and the respective transcriptomes analysed by RNA-seq. The differential expression (DE) of genes revealed a strong immune activation in DCs of the susceptible line, although an inherent immune supremacy was shown by the resistant line, including a significant expression of tumour-suppressor miRNA, gga-mir-124a, in line 61 control birds. Enrichment analysis of DE genes revealed high expression of an oncogenic transcription factor, AP-1, in the susceptible line following MDV challenge. This research highlights genes and pathways that may play a role in DCs in determining resistance or susceptibility to MDV infection.
Keywords: AP-1; Marek’s disease virus; RNAseq; chicken; dendritic cells; disease resistance; mir-124a; transcriptome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Davison T.F., Kaiser P. Immunity to Marek’s disease. In: Davison F., Nair V., editors. Marek’s Disease: An Evolving Problem. Elsevier Academic Press; London, UK: 2004. pp. 126–139.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

