A MWCNTs-COOH/PSS nanocomposite-modified screen-printed electrode for the determination of synthetic phenolic antioxidants by HPLC with amperometric detection
- PMID: 36422711
- PMCID: PMC9691489
- DOI: 10.1007/s00604-022-05552-7
A MWCNTs-COOH/PSS nanocomposite-modified screen-printed electrode for the determination of synthetic phenolic antioxidants by HPLC with amperometric detection
Abstract
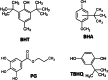
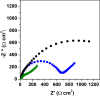
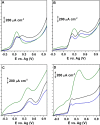
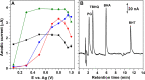
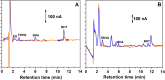
New sensing platforms based on screen-printed carbon electrodes modified with composites based on polystyrene sulfonate and oxidized multi-walled carbon nanotubes (PSS/MWCNTs-COOH/SPCE) have been used to develop a novel HPLC method with electrochemical detection (ECD) for the determination of the most used synthetic phenolic antioxidants in cosmetics: butylhydroxytoluene (BHT), butylhydroxyanisole (BHA), tert-butylhydroquinone (TBHQ) and propyl gallate (PG). Optimal separation conditions were achieved using methanol: 0.10 mol L-1 acetate solution at pH 6 as mobile phase with a gradient elution program from 60 to 90% of methanol percentage in 15 min. The electrochemical detection was carried out in amperometric mode using the PSS/MWCNTs-COOH/SPCE at + 0.80 V vs. Ag. Under these optimal separation and detection conditions, the limits of detection (LOD) were between 0.11 and 0.25 mg L-1. These LOD values were better, especially for BHT, than those previously published in other HPLC methods. Linear ranges from 0.37 mg L-1, 0.83 mg L-1, 0.69 mg L-1 and 0.56 mg L-1 to 10 mg L-1 were obtained for PG, TBHQ, BHA and BHT, respectively. RSD values equal or lower than 5% and 8% were achieved for repeatability and reproducibility, respectively. The HPLC-ECD method was successfully applied to analyze different cosmetic samples. Recovery values within 83-109% were obtained in the validation studies.
Keywords: Carbon nanotubes; Electrochemical detection; HPLC; Nanocomposites; Polystyrene sulfonate; Synthetic phenolic antioxidants.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Khan MK, Paniwnyk L, Hassan S (2019) Polyphenols as natural antioxidants: sources, extraction and applications in food, cosmetics and drugs. In: Li Y, Chemat F (eds) Plant based “green chemistry 2.0”. Green Chemistry and Sustainable Technology. Springer, Singapore. 10.1007/978-981-13-3810-6_8
-
- Hoang HT, Moon JY, Lee YC. Natural antioxidants from plant extracts in skincare cosmetics: recent applications, challenges and perspectives. Cosmetics. 2021;8:106. doi: 10.3390/cosmetics8040106. - DOI
-
- Alvarez-Rivera G, Llompart M, Lores M, Garcia-Jares C (2018) Preservatives in cosmetics: regulatory aspects and analytical methods. In: Salvador A, Chisvert A (eds) Anal Cosmet Prod, 2nd edn. Elsevier, pp 175–224. 10.1016/B978-0-444-63508-2.00009-6
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
