Small Peptides in the Detection of Mycotoxins and Their Potential Applications in Mycotoxin Removal
- PMID: 36422969
- PMCID: PMC9698726
- DOI: 10.3390/toxins14110795
Small Peptides in the Detection of Mycotoxins and Their Potential Applications in Mycotoxin Removal
Abstract
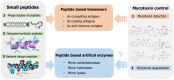
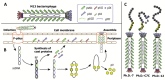
Mycotoxins pose significant risks to humans and livestock. In addition, contaminated food- and feedstuffs can only be discarded, leading to increased economic losses and potential ecological pollution. Mycotoxin removal and real-time toxin level monitoring are effective approaches to solve this problem. As a hot research hotspot, small peptides derived from phage display peptide libraries, combinatorial peptide libraries, and rational design approaches can act as coating antigens, competitive antigens, and anti-immune complexes in immunoassays for the detection of mycotoxins. Furthermore, as a potential approach to mycotoxin degradation, small peptides can mimic the natural enzyme catalytic site to construct artificial enzymes containing oxidoreductases, hydrolase, and lyase activities. In summary, with the advantages of mature synthesis protocols, diverse structures, and excellent biocompatibility, also sharing their chemical structure with natural proteins, small peptides are widely used for mycotoxin detection and artificial enzyme construction, which have promising applications in mycotoxin degradation. This paper mainly reviews the advances of small peptides in the detection of mycotoxins, the construction of peptide-based artificial enzymes, and their potential applications in mycotoxin control.
Keywords: artificial enzymes; mycotoxin control; mycotoxin detection; mycotoxin removal; small peptides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Development and comparison of mimotope-based immunoassays for the analysis of fumonisin B1.Anal Bioanal Chem. 2019 Oct;411(26):6801-6811. doi: 10.1007/s00216-019-02068-7. Epub 2019 Aug 17. Anal Bioanal Chem. 2019. PMID: 31422432
-
Phage displayed mimotope peptide-based immunosensor for green and ultrasensitive detection of mycotoxin deoxynivalenol.J Pharm Biomed Anal. 2019 May 10;168:94-101. doi: 10.1016/j.jpba.2019.01.051. Epub 2019 Jan 31. J Pharm Biomed Anal. 2019. PMID: 30802751
-
Mimotopes for Mycotoxins Diagnosis Based on Random Peptides or Recombinant Antibodies from Phage Library.Molecules. 2021 Dec 17;26(24):7652. doi: 10.3390/molecules26247652. Molecules. 2021. PMID: 34946736 Free PMC article. Review.
-
Toxicant substitutes in immunological assays for mycotoxins detection: A mini review.Food Chem. 2021 May 15;344:128589. doi: 10.1016/j.foodchem.2020.128589. Epub 2020 Nov 16. Food Chem. 2021. PMID: 33246689 Review.
-
Mimotope-Based Immunoassays for the Rapid Analysis of Mycotoxin: A Review.J Agric Food Chem. 2021 Oct 13;69(40):11743-11752. doi: 10.1021/acs.jafc.1c04169. Epub 2021 Sep 28. J Agric Food Chem. 2021. PMID: 34583509 Review.
Cited by
-
Stability of ACE2 Peptide Mimetics and Their Implications on the Application for SARS-CoV2 Detection.Biosensors (Basel). 2023 Apr 13;13(4):473. doi: 10.3390/bios13040473. Biosensors (Basel). 2023. PMID: 37185548 Free PMC article.
-
Advances in phage display based nano immunosensors for cholera toxin.Front Immunol. 2023 Sep 13;14:1224397. doi: 10.3389/fimmu.2023.1224397. eCollection 2023. Front Immunol. 2023. PMID: 37781379 Free PMC article. Review.
-
A NADPH-Dependent Aldo/Keto Reductase Is Responsible for Detoxifying 3-Keto-Deoxynivalenol to 3-epi-Deoxynivalenol in Pelagibacterium halotolerans ANSP101.Foods. 2024 Mar 29;13(7):1064. doi: 10.3390/foods13071064. Foods. 2024. PMID: 38611368 Free PMC article.
-
Ochratoxin A Degradation and Stress Response Mechanism of Brevundimonas naejangsanensis ML17 Determined by Transcriptomic Analysis.Foods. 2024 Nov 21;13(23):3732. doi: 10.3390/foods13233732. Foods. 2024. PMID: 39682804 Free PMC article.
References
-
- Iqbal S.Z. Mycotoxins in food, recent development in food analysis and future challenges; A review. Curr. Opin. Food Sci. 2021;42:237–247. doi: 10.1016/j.cofs.2021.07.003. - DOI
-
- Khodaei D., Javanmardi F., Khaneghah A.M. The global overview of the occurrence of mycotoxins in cereals: A three-year survey. Curr. Opin. Food Sci. 2021;39:36–42. doi: 10.1016/j.cofs.2020.12.012. - DOI
-
- Awuchi C.G., Ondari E.N., Ogbonna C.U., Upadhyay A.K., Baran K., Okpala C.O.R., Korzeniowska M., Guiné R.P.F. Mycotoxins affecting animals, foods, humans, and plants: Types, occurrence, toxicities, action mechanisms, prevention, and detoxification strategies-A revisit. Foods. 2021;10:1279. doi: 10.3390/foods10061279. - DOI - PMC - PubMed
-
- Dey D.K., Kang J.I., Bajpai V.K., Kim K., Lee H., Sonwal S., Simal-Gandara J., Xiao J., Ali S., Huh Y.S., et al. Mycotoxins in food and feed: Toxicity, preventive challenges, and advanced detection techniques for associated diseases. Crit. Rev. Food Sci. Nutr. 2022;62:1–22. doi: 10.1080/10408398.2022.2059650. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

