Detoxification of the Mycotoxin Citrinin by a Manganese Peroxidase from Moniliophthora roreri
- PMID: 36422974
- PMCID: PMC9693499
- DOI: 10.3390/toxins14110801
Detoxification of the Mycotoxin Citrinin by a Manganese Peroxidase from Moniliophthora roreri
Abstract
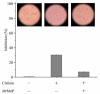
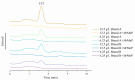
Citrinin (CIT) is a mycotoxin found in foods and feeds and most commonly discovered in red yeast rice, a food additive made from ordinary rice by fermentation with Monascus. Currently, no enzyme is known to be able to degrade CIT effectively. In this study, it was discovered that manganese peroxidase (MrMnP) from Moniliophthora roreri could degrade CIT. The degradation appeared to be fulfilled by a combination of direct and indirect actions of the MrMnP with the CIT. Pure CIT, at a final concentration of 10 mg/L, was completely degraded by MrMnP within 72 h. One degradation product was identified to be dihydrocitrinone. The toxicity of the CIT-degradation product decreased, as monitored by the increased survival rate of the Caco-2 cells incubated with MrMnP-treated CIT. In addition, MrMnP could degrade CIT (with a starting concentration of up to 4.6 mg/L) completely contaminated in red yeast rice. MrMnP serves as an excellent candidate enzyme for CIT detoxification.
Keywords: citrinin; detoxification; manganese peroxidase; mycotoxin; red yeast rice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

