Confirmation Using Triple Quadrupole and High-Resolution Mass Spectrometry of a Fatal Canine Neurotoxicosis following Exposure to Anatoxins at an Inland Reservoir
- PMID: 36422978
- PMCID: PMC9696769
- DOI: 10.3390/toxins14110804
Confirmation Using Triple Quadrupole and High-Resolution Mass Spectrometry of a Fatal Canine Neurotoxicosis following Exposure to Anatoxins at an Inland Reservoir
Abstract
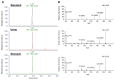
Cyanobacterial blooms are often associated with the presence of harmful natural compounds which can cause adverse health effects in both humans and animals. One family of these compounds, known as anatoxins, have been linked to the rapid deaths of cattle and dogs through neurotoxicological action. Here, we report the findings resulting from the death of a dog at a freshwater reservoir in SW England. Poisoning was rapid following exposure to material at the side of the lake. Clinical signs included neurological distress, diaphragmatic paralysis and asphyxia prior to death after 45 min of exposure. Analysis by HILIC-MS/MS of urine and stomach content samples from the dog revealed the detection of anatoxin-a and dihydroanatoxin-a in both samples with higher concentrations of the latter quantified in both matrices. Detection and quantitative accuracy was further confirmed with use of accurate mass LC-HRMS. Additional anatoxin analogues were also detected by LC-HRMS, including 4-keto anatoxin-a, 4-keto-homo anatoxin-a, expoxy anatoxin-a and epoxy homo anatoxin-a. The conclusion of neurotoxicosis was confirmed with the use of two independent analytical methods showing positive detection and significantly high quantified concentrations of these neurotoxins in clinical samples. Together with the clinical signs observed, we have confirmed that anatoxins were responsible for the rapid death of the dog in this case.
Keywords: anatoxin-a; cyanobacteria; cyanotoxins; dihydroanatoxin-a; dog poisoning; reservoir.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Chorus I., Welker M. Toxic Cyanobacteria in Water—Second Edition. CRC Press; Boca Raton, FL, USA: 2021.
-
- Kuiper-Goodman T., Falconer I., Fitzgerald J. Human Health Aspects. In: Bartram J., editor. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management. CRC Press; Boca Raton, FL, USA: 1999. pp. 125–161.
-
- Metcalf J.S., Codd G.A. Ecology of Cyanobacteria II. Springer; Dordrecht, The Netherlands: 2012. Cyanotoxins; pp. 651–675.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

