Extracellular Vesicles from Bothrops jararaca Venom Are Diverse in Structure and Protein Composition and Interact with Mammalian Cells
- PMID: 36422980
- PMCID: PMC9698812
- DOI: 10.3390/toxins14110806
Extracellular Vesicles from Bothrops jararaca Venom Are Diverse in Structure and Protein Composition and Interact with Mammalian Cells
Abstract
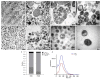
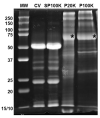
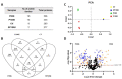
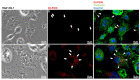
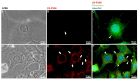
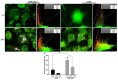
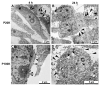
Snake venoms are complex cocktails of non-toxic and toxic molecules that work synergistically for the envenoming outcome. Alongside the immediate consequences, chronic manifestations and long-term sequelae can occur. Recently, extracellular vesicles (EVs) were found in snake venom. EVs mediate cellular communication through long distances, delivering proteins and nucleic acids that modulate the recipient cell's function. However, the biological roles of snake venom EVs, including possible cross-organism communication, are still unknown. This knowledge may expand the understanding of envenoming mechanisms. In the present study, we isolated and characterized the EVs from Bothrops jararaca venom (Bj-EVs), giving insights into their biological roles. Fresh venom was submitted to differential centrifugation, resulting in two EV populations with typical morphology and size range. Several conserved EV markers and a subset of venom related EV markers, represented mainly by processing enzymes, were identified by proteomic analysis. The most abundant protein family observed in Bj-EVs was 5'-nucleotidase, known to be immunosuppressive and a low abundant and ubiquitous toxin in snake venoms. Additionally, we demonstrated that mammalian cells efficiently internalize Bj-EVs. The commercial antibothropic antivenom partially recognizes Bj-EVs and inhibits cellular EV uptake. Based on the proteomic results and the in vitro interaction assays using macrophages and muscle cells, we propose that Bj-EVs may be involved not only in venom production and processing but also in host immune modulation and long-term effects of envenoming.
Keywords: 5′-nucleotidase; extracellular vesicles; snake venom.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

