The Influence of Micronutrient Trace Metals on Microcystis aeruginosa Growth and Toxin Production
- PMID: 36422986
- PMCID: PMC9694995
- DOI: 10.3390/toxins14110812
The Influence of Micronutrient Trace Metals on Microcystis aeruginosa Growth and Toxin Production
Abstract
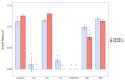
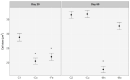
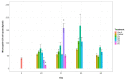
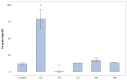
Microcystis aeruginosa is a widespread cyanobacteria capable of producing hepatotoxic microcystins. Understanding the environmental factors that influence its growth and toxin production is essential to managing the negative effects on freshwater systems. Some micronutrients are important cofactors in cyanobacterial proteins and can influence cyanobacterial growth when availability is limited. However, micronutrient requirements are often species specific, and can be influenced by substitution between metals or by luxury uptake. In this study, M. aeruginosa was grown in modified growth media that individually excluded some micronutrients (cobalt, copper, iron, manganese, molybdenum) to assess the effect on growth, toxin production, cell morphology and iron accumulation. M. aeruginosa growth was limited when iron, cobalt and manganese were excluded from the growth media, whereas the exclusion of copper and molybdenum had no effect on growth. Intracellular microcystin-LR concentrations were variable and were at times elevated in treatments undergoing growth limitation by cobalt. Intracellular iron was notably higher in treatments grown in cobalt-deplete media compared to other treatments possibly due to inhibition or competition for transporters, or due to irons role in detoxifying reactive oxygen species (ROS).
Keywords: cyanobacteria; growth limitation; microcystin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Assessing the importance of cobalt as a micronutrient for freshwater cyanobacteria.J Phycol. 2022 Feb;58(1):71-79. doi: 10.1111/jpy.13216. Epub 2021 Nov 10. J Phycol. 2022. PMID: 34633686
-
Toxins from harmful algal blooms: How copper and iron render chalkophore a predictor of microcystin production.Water Res. 2023 Oct 1;244:120490. doi: 10.1016/j.watres.2023.120490. Epub 2023 Aug 14. Water Res. 2023. PMID: 37659180
-
A Review of the Effect of Trace Metals on Freshwater Cyanobacterial Growth and Toxin Production.Toxins (Basel). 2019 Nov 5;11(11):643. doi: 10.3390/toxins11110643. Toxins (Basel). 2019. PMID: 31694295 Free PMC article. Review.
-
Trace element uptake by L-cells as a function of trace elements in a synthetic growth medium.J Cell Physiol. 1979 Oct;101(1):57-65. doi: 10.1002/jcp.1041010108. J Cell Physiol. 1979. PMID: 541353
-
Trace metals in human and animal health.J Hum Nutr. 1981 Feb;35(1):37-48. doi: 10.3109/09637488109143484. J Hum Nutr. 1981. PMID: 7009733 Review. No abstract available.
References
-
- Sciuto K., Moro I. Cyanobacteria: The Bright and Dark Sides of a Charming Group. Biodivers. Conserv. 2015;24:711–738. doi: 10.1007/s10531-015-0898-4. - DOI
-
- Dignum M., Matthijs H.C.P., Pel R., Laanbroek H.J., Mur L.R. Nutrient Limitation of Freshwater Cyanobacteria. In: Huisman J., Matthijs H.C.P., Visser P.M., editors. Harmful Cyanobacteria. Aquatic Ecology Series. Volume 3. Springer; Dordrecht, The Netherlands: 2005. pp. 65–86. - DOI
-
- Paerl H.W., Fulton R.S. In: Ecology of Harmful Algae. Graneli E., Turner J., editors. Springer; New York, NY, USA: 2006. pp. 95–111. - DOI
-
- North R.L., Guildford S.J., Smith R.E.H., Havens S.M., Twiss M.R. Evidence for Phosphorus, Nitrogen, and Iron Colimitation of Phytoplankton Communities in Lake Erie. Limnol. Oceanogr. 2007;52:315–328. doi: 10.4319/lo.2007.52.1.0315. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

