The tryptophan-metabolizing enzyme indoleamine 2,3-dioxygenase 1 regulates polycystic kidney disease progression
- PMID: 36422996
- PMCID: PMC9870090
- DOI: 10.1172/jci.insight.154773
The tryptophan-metabolizing enzyme indoleamine 2,3-dioxygenase 1 regulates polycystic kidney disease progression
Abstract
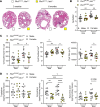
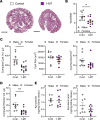
Autosomal dominant polycystic kidney disease (ADPKD), the most common monogenic nephropathy, is characterized by phenotypic variability that exceeds genic effects. Dysregulated metabolism and immune cell function are key disease modifiers. The tryptophan metabolites, kynurenines, produced through indoleamine 2,3-dioxygenase 1 (IDO1), are known immunomodulators. Here, we study the role of tryptophan metabolism in PKD using an orthologous disease model (C57BL/6J Pkd1RC/RC). We found elevated kynurenine and IDO1 levels in Pkd1RC/RC kidneys versus wild type. Further, IDO1 levels were increased in ADPKD cell lines. Genetic Ido1 loss in Pkd1RC/RC animals resulted in reduced PKD severity, as measured by cystic index and percentage kidney weight normalized to body weight. Consistent with an immunomodulatory role of kynurenines, Pkd1RC/RC;Ido1-/- mice presented with significant changes in the cystic immune microenvironment (CME) versus controls. Kidney macrophage numbers decreased and CD8+ T cell numbers increased, both known PKD modulators. Also, pharmacological IDO1 inhibition in Pkd1RC/RC mice and kidney-specific Pkd2-knockout mice with rapidly progressive PKD resulted in less severe PKD versus controls, with changes in the CME similar to those in the genetic model. Our data suggest that tryptophan metabolism is dysregulated in ADPKD and that its inhibition results in changes to the CME and slows disease progression, making IDO1 a therapeutic target for ADPKD.
Keywords: Amino acid metabolism; Cellular immune response; Monogenic diseases; Nephrology.
Figures



References
-
- United States Renal Data System. 2018 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/usrds/prio... Accessed December 13, 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

